Figures & data
Figure 1 Der Simonian and Laird relative risks (random effects) plot of beta-blocker versus placebo in the subgroup of elderly patients with heart failure. Point estimates and 95% CIs represented next to box plot.
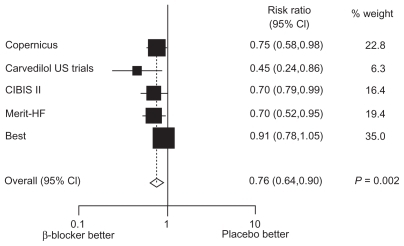
Figure 2 Time to all-cause mortality or cardiovascular hospital admission (primary endpoint) in SENIORS.
Abbreviations: NEB, nebivolol; PL, placebo. Copyright© 2005. Modified with permission from Oxford University Press. Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225.Citation47
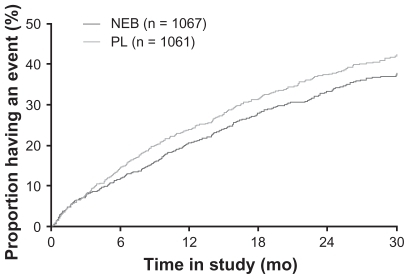
Figure 3 Prespecified sub-group analysis of SENIORS study. No interaction was found in subgroups with respect to the primary end-point.
*Number of events per 100 patient-years of follow-up at risk. **P-value for interaction: age and left ventricular ejection fraction considered as continuous variables.
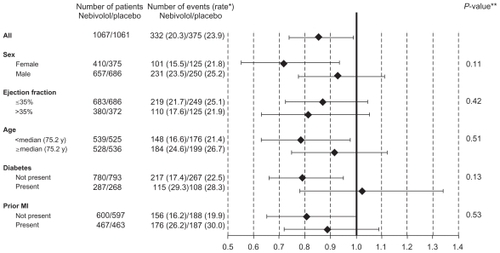
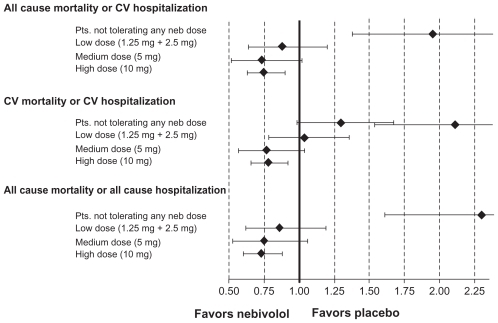
Figure 4 Tolerability profile of nebivolol in SENIORS.
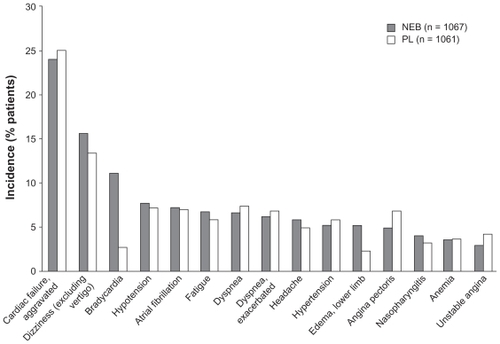
Figure 5 Time to all-cause mortality in patients aged <75.2 years (median age) with left ventricular ejection fraction (LVEF) ≤35% in SENIORS. The hazard ratio was 0.62 (95% CI: 0.43, 0.89; P = 0.011).
Abbreviations: NEB, nebivolol; PL, placebo. Copyright© 2006. Modified with permission from Wolters Kluwer. Moen MD, Wagstaff AJ. Nebivolol: a review of its use in the management of hypertension and chronic heart failure. Drugs. 2006;66(10): 1389–1409.Citation27
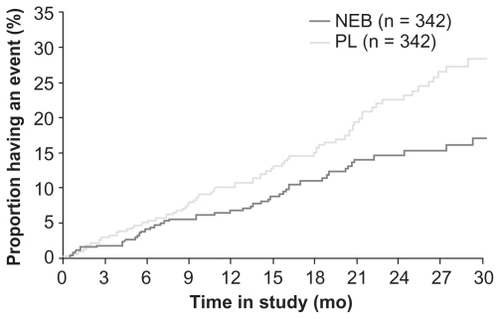
Figure 6 Hazard ratio plots (with 95% CIs) for total mortality for comparable patient subgroups from the four main beta-blockers mortality trials, ie, SENIORS [nebivolol]; COPERNICUS [carvedilol], MERIT-HF [metoprolol] and CIBIS II [bisoprolol], using data derived from the trial reports. These data are from published patient subgroups reported by the authors themselves for each trial, and the criteria, therefore, differ between trials. The reported patient age subgroups chosen here are those most similar to each other across the four trials. For nebivolol, this is left ventricular ejection fraction (LVEF) ≤35% and age less than median (70–75.2 years); for carvedilol, LVEF ≤ 25% and age ≥65 years; for metoprolol LVEF ≤ 40% and age >69 years; and for bisoprolol LVEF ≤ 35% and age ≥71 years. Copyright© 2005. Modified with permission from Coats AJS. Coats AJS, The modern tailored management of chronic heart failure: SENIORS. Proceedings of the Annual Congress of the European Society of Cardiology; 2005 Sep 3–7; Stockholm.Citation52
![Figure 6 Hazard ratio plots (with 95% CIs) for total mortality for comparable patient subgroups from the four main beta-blockers mortality trials, ie, SENIORS [nebivolol]; COPERNICUS [carvedilol], MERIT-HF [metoprolol] and CIBIS II [bisoprolol], using data derived from the trial reports. These data are from published patient subgroups reported by the authors themselves for each trial, and the criteria, therefore, differ between trials. The reported patient age subgroups chosen here are those most similar to each other across the four trials. For nebivolol, this is left ventricular ejection fraction (LVEF) ≤35% and age less than median (70–75.2 years); for carvedilol, LVEF ≤ 25% and age ≥65 years; for metoprolol LVEF ≤ 40% and age >69 years; and for bisoprolol LVEF ≤ 35% and age ≥71 years. Copyright© 2005. Modified with permission from Coats AJS. Coats AJS, The modern tailored management of chronic heart failure: SENIORS. Proceedings of the Annual Congress of the European Society of Cardiology; 2005 Sep 3–7; Stockholm.Citation52](/cms/asset/e6cfdbb8-b0cd-4ea8-8197-717512f55764/dcia_a_4482_f0006_b.jpg)
Figure 7 Primary and secondary outcomes (HR with 95% CI) in patients receiving placebo versus nebivolol at different maintenance doses.