Figures & data
Figure 1 Overall study design.
Abbreviation: PRM, prolonged-release melatonin.
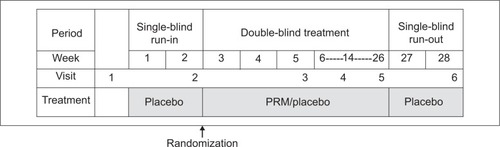
Figure 2 Overall study patient disposition.
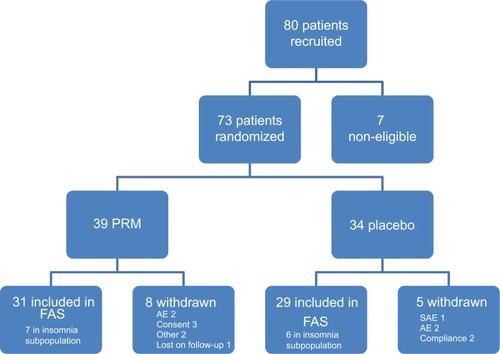
Table 1 Changes from baseline in cognitive outcome, as assessed by ADAS-Cog, IADL, and MMSE after 24 weeks of treatment
Figure 3 Cognitive assessments.
Abbreviations: AD, Alzheimer’s disease; ADAS-Cog, AD Assessment Scale–Cognition; ANCOVA, analysis of covariance; FAS, full analysis set; IADL, Instrumental Activities of Daily Living; MMRM, mixed-effects model for repeated measures; MMSE, Mini–Mental State Examination; PRM, prolonged-release melatonin; PSQI, Pittsburgh Sleep Quality Index; SEM, standard error of the mean.
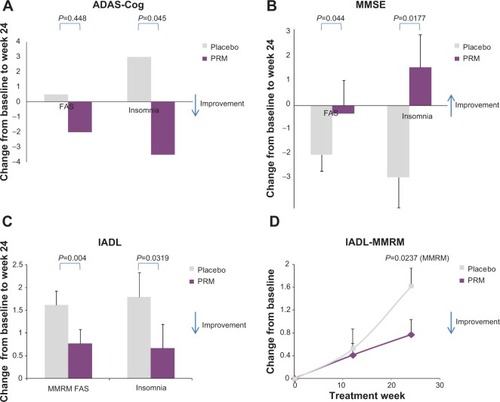
Table 2 Effects of PRM and placebo on PSQI global score and items, by treatment and period – FAS population
Table 3 Effects of PRM and placebo on PSQI global score and items, by treatment and period – insomnia comorbidity subpopulation
Figure 4 Sleep assessments.
Abbreviations: ANCOVA, analysis of covariance; C4, component 4; FAS, full analysis set; PRM, prolonged-release melatonin; PSQI, Pittsburgh Sleep Quality Index.
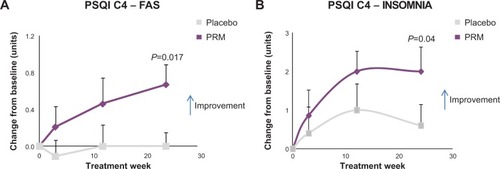
Table S1 Baseline characteristics of the study population
Table S2 The change in sleep quality measured from sleep diary parameters, between baseline and 12 weeks of treatment (FAS)
Table S3 Number (%) of patients who had an adverse event (AE), in the safety population
Table S4 Overall adverse events and most frequent events, by system organ class and preferred term, in >5% of patients (two patients) in any cohort, and drug-related AEs
