Figures & data
Table 1 Demographic and clinical characteristics of the pooled population
Table 2 AEs by category with saxagliptin versus placebo
Figure 1 IRRs of AEs by (A) category and (B) type with saxagliptin 5 mg versus placebo.
Abbreviations: AE, adverse event; CI, confidence interval; D/C, discontinuation; IRR, incidence rate ratio.
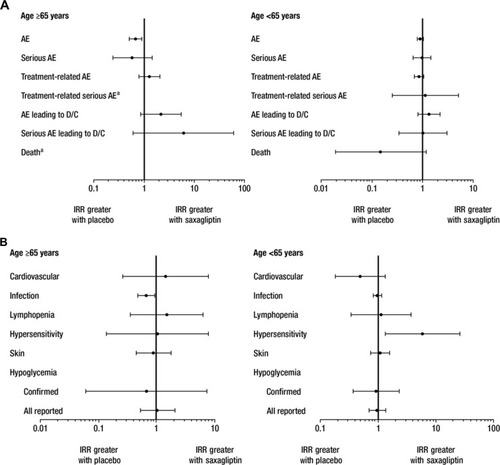
Table 3 AEs with an IR ≥5 events per 100 person-years in any groupTable Footnotea
Table 4 AEs of special interest with saxagliptin 5 mg versus placebo
