Figures & data
Table 1 Study inclusion at screening and baseline
Table 2 Study exclusion criteria
Table 3 Baseline demographic and clinical characteristics for all randomly assigned patients
Figure 1 CONSORT (consolidated standards of reporting trials) diagram showing the disposition of participants. Group 1, alfuzosin 10 mg monotherapy; group 2, alfuzosin 10 mg combined antihypertensive therapy.
Figure 2 Mean supine blood pressure and HR values stratified by normotensive and untreated hypertensive status in group 1 and controlled hypertensive and uncontrolled hypertensive status in group 2 at baseline and at endpoint. (A) Systolic blood pressure, (B) diastolic blood pressure, and (C) HR.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; min, minute.
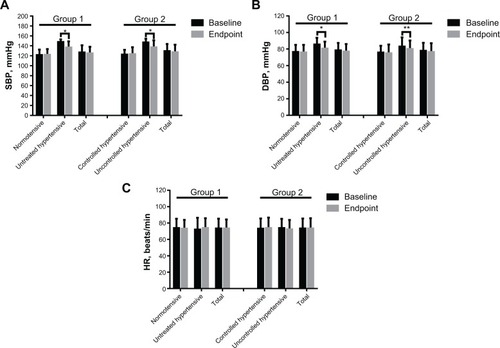
Figure 3 Mean change in supine blood pressure and HR from baseline to endpoint stratified by normotensive and untreated hypertensive status in group 1 and controlled hypertensive and uncontrolled hypertensive status in group 2. (A) Mean change in SBP; (B) mean change in DBP, and (C) mean change in HR.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; min, minute.
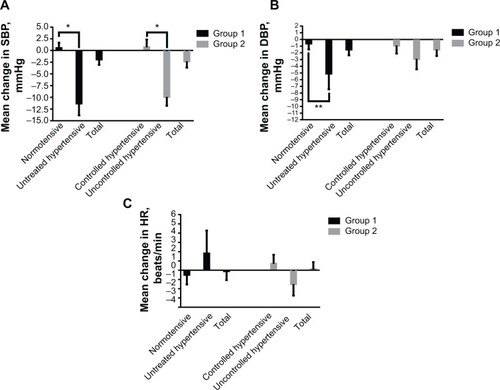
Figure 4 Mean change in efficacy measures from baseline to endpoint in groups 1 and 2. (A) Total IPSS score, (B) IPSS-QoL, (C), Qmax, (D), Qave, (E), Vvoid, and (F) PVR.
Abbreviations: IPSS, International Prostate Symptom Score; PVR, post-voiding residual volume; Qave, average flow rate; Qmax, maximum flow rate; QoL, quality of life; Vvoid, voided volume.
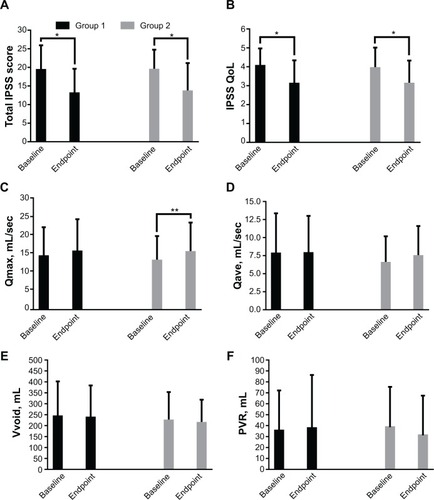
Table 4 Mean change in efficacy measures from baseline to endpoint for groups 1 and 2
Table 5 Withdrawals and adverse events from therapy
