Figures & data
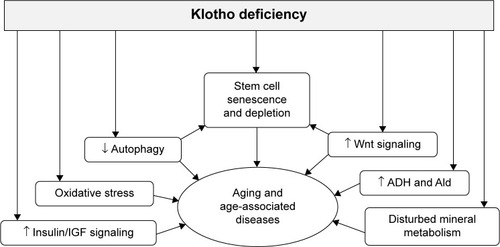
Figure 1 Schematic diagram of membrane klotho and secreted klotho.
Notes: Membrane form of klotho transcript arises from the klotho gene. Secreted klotho arises from an alternative RNA splicing. The internal splice donor site is in exon 3 of the klotho gene. The resultant alternatively spliced transcript contains insertion of 50 bp after exon 3 (gray), with an in-frame translation stop codon at the end. The product is released into the blood circulation. On the other hand, membrane klotho protein encoded by the membrane form of klotho transcript is a single-pass transmembrane protein. The extracellular domain of membrane klotho containing KL1 and KL2 repeats is shed and cleaved by α/β-secretases, and released into the circulating stream. Therefore, there are two forms of klotho protein in the circulation. One is derived from cleavage of the extracellular domain of membrane klotho called soluble klotho, a well-known active form. The other is the secreted membrane protein, which is directly derived from an alternatively spliced klotho transcript, but its function is largely unknown.
Abbreviation: mRNA, messenger RNA.
Abbreviation: mRNA, messenger RNA.
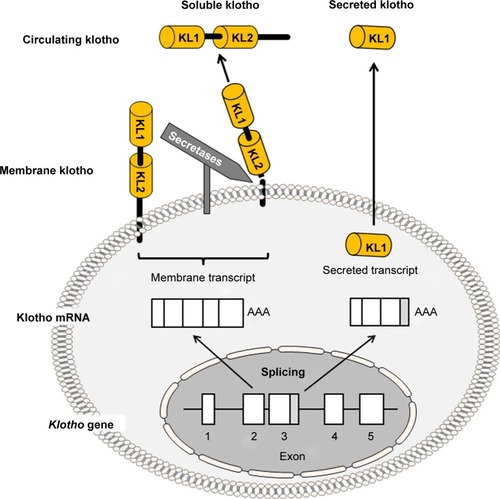
Figure 2 Potential effects of klotho deficiency on stem cell depletion and the aging process.
Notes: Klotho deficiency stimulates the Wnt signaling pathway to directly accelerate aging or indirectly induce stem cell depletion. Klotho deficiency downregulates autophagy, and low autophagy consequently accelerates aging and induces stem cell senescence and depletion. Klotho deficiency also activates the Wnt signaling pathway, and the resulting excessive Wnt signal activity also induces stem cell senescence and depletion. Both low autophagy and high Wnt signaling activity may directly accelerate aging. Klotho deficiency in turn depresses insulin/IGF signaling activity, disrupts mineral-metabolism homeostasis, and amplifies oxidative stress. Klotho deficiency is also associated with higher levels of blood antidiuretic hormone (ADH) and aldosterone (Ald). All of these effects accelerate the aging process and trigger or exacerbate age-associated diseases. Conceivably, elevation of klotho can interrupt klotho deficiency-induced pathologic processes, slow down aging processes, and attenuate age-associated diseases.