Figures & data
Table 1 Demographic and clinical characteristics of the 1,426 patients of the intention-to-treat population of the two studies pooled together
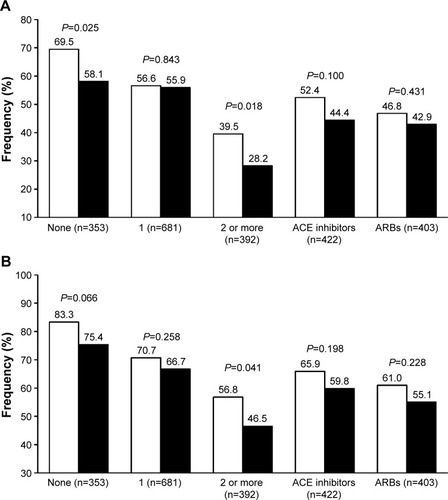
Figure 1 Percentage of normalized patients according to different thresholds.
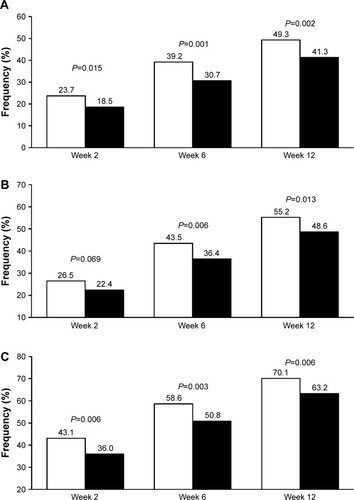
Table 2 Blood pressure response to olmesartan monotherapy in elderly patients with systolic and/or diastolic hypertension or isolated systolic hypertension in different open-label or double-blind randomized studies
Table 3 Percentage of normalized and normalized or responder patients after 12 weeks of treatment with olmesartan medoxomil 10–40 mg (n=712) or ramipril 2.5–10 mg (n=714), according to sex, age and 10-year cardiovascular risk category (low-moderate: <5% and high-very high: ≥5%)
Figure 2 Percentage of normalized patients.
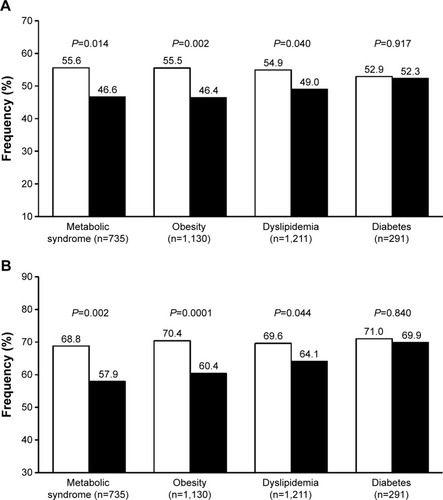
Figure 3 Percentage of normalized patients.
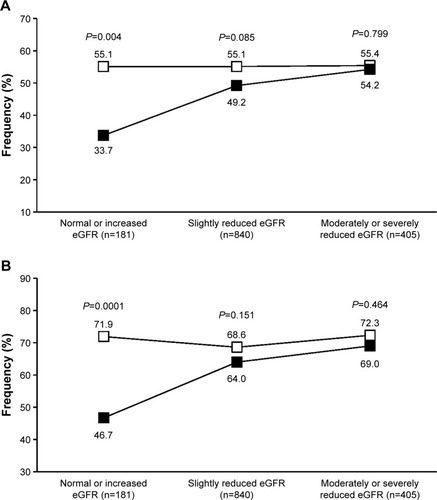
Figure 4 Percentage of normalized patients.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker.