Figures & data
Figure 1 Schematic diagram of the primary sequence of IAPP.
Abbreviation: IAPP, islet amyloid polypeptide.
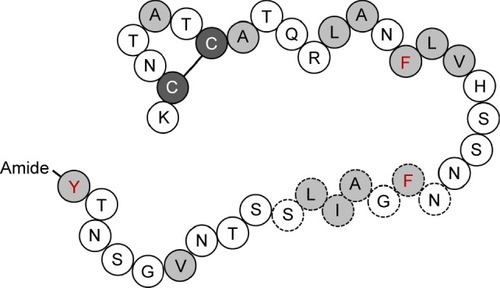
Figure 2 List of genetic mutations of IAPP determined from in silico studies, and naturally occurring (eg, S20G) mutations.
Abbreviation: IAPP, islet amyloid polypeptide.
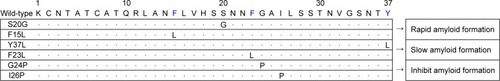
Figure 3 Alignment of IAPP amino acid sequences from different species.
Abbreviation: IAPP, islet amyloid polypeptide.
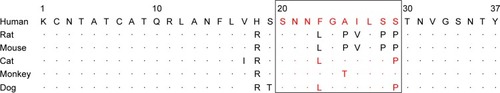
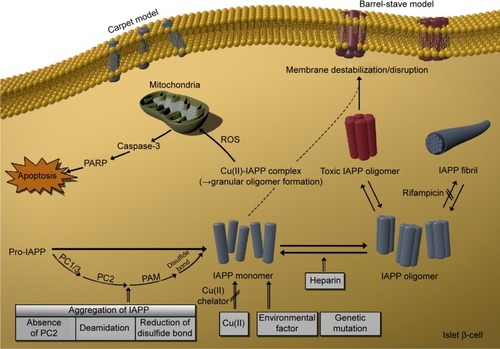
Figure 4 Causative factors of toxic oligomer formation.
Abbreviations: IAPP, islet amyloid polypeptide; PARP, poly (ADP-ribose) polymerase; ROS, reactive oxygen species; PC1/3, proprotein convertase 1/3; PC2, proprotein convertase 2; PAM, peptidylglycine α-amidating monooxygenase; Cu(II), copper (II) ion.