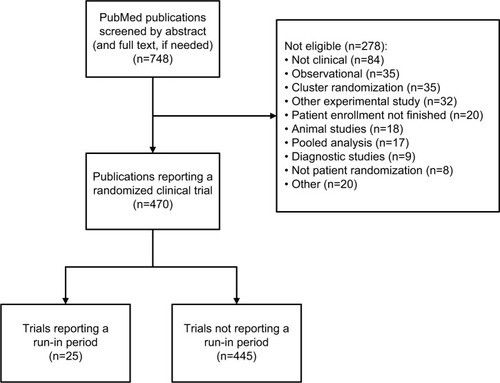
Figures & data
Table 1 General characteristics of randomized clinical trials with and without run-in periodsTable Footnotea
Table 2 Characteristics of randomized clinical trials with run-in periods
Table 3 Characteristics of the run-in period in the 25 trials reporting such a period
Table S1 Industry status in trials with and without run-in periodsTable Footnotea