Figures & data
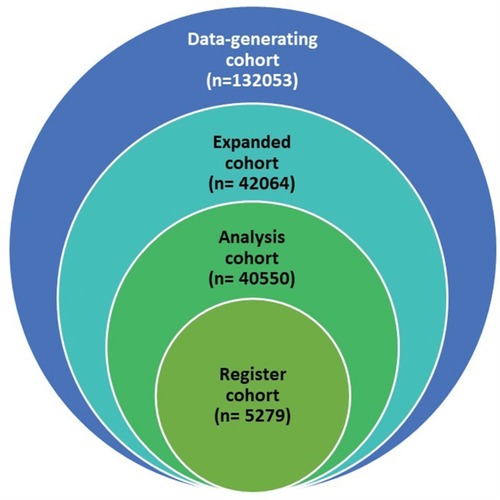
Table 1 Overview Of Definitions And Rationale For The Three Study Cohorts Of The ERASE Project
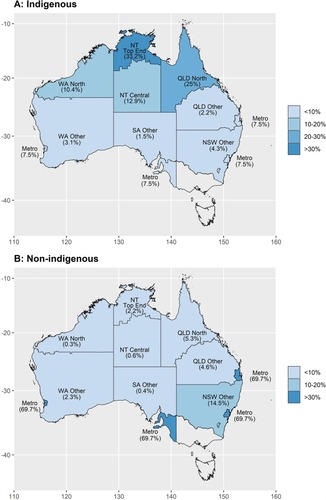
Table 2 Cumulative Frequencies Of Persons Included In The Expanded Cohort By Data Source
Table 3 Cumulative Frequencies Of First-Ever Acute Rheumatic Fever (ARF) Diagnosis Dates In The Expanded Cohort, By Data Source
Table 4 Cumulative Frequencies Of Rheumatic Heart Disease (RHD) Onset Dates In The Expanded Cohort, By Data Source
Figure 2 Age distribution of cases at time of initial (A). Acute rheumatic fever (ARF) and (B). Rheumatic heart disease (RHD) diagnoses, by Indigenous status (2001–2017, mid-year).
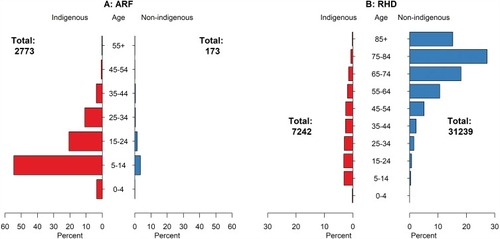
Table 5 Descriptive Profile Of Study Cohorts For Patients Under 60 Years At The Time Of First ARF Or RHD Diagnosis, N (%)
Data Availability
The ERASE database cannot be shared publicly. Australian-based researchers can apply to the ERASE Project team with a proposal to analyze a pertinent research question using the ERASE Project data, subject to internal and ethics approval of the investigator and their research plans.