Figures & data
Table 1 Summary of PIVOTAL TrialCitation15 Treatment Strategies Emulated in DOPPS
Figure 1 Illustration of longitudinal data collection and hypothesized relationships.
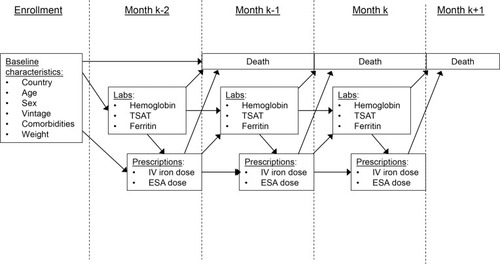
Table 2 Summary of Step 1 Models and Covariates
Figure 2 Flow diagram with PIVOTAL trialCitation15 exclusion criteria.
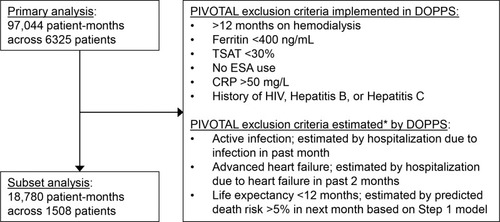
Table 3 Summary of Baseline Patient Characteristics in the DOPPS (by Type of Analysis) and the PIVOTAL TrialCitation15 (by Treatment Group)
Figure 3 Comparison of proactive high-dose vs reactive low-dose IV iron treatment strategy over 12 months using the parametric g-formula. High-dose and low-dose strategies defined by PIVOTAL trialCitation15 protocol as described in ; Outcomes: (A) all-cause mortality, (B) hemoglobin, (C) serum ferritin, (D) TSAT, (E) ESA dose, (F) IV iron dose.
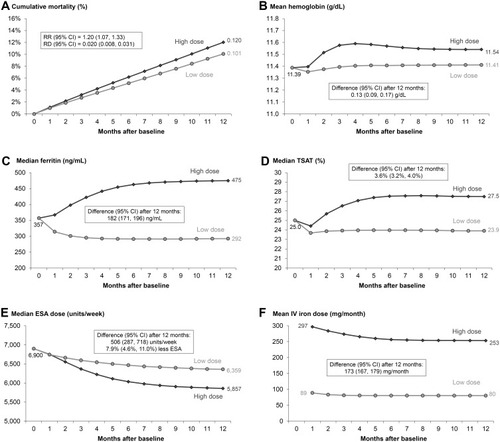
Figure 4 Comparison of proactive high-dose vs reactive low-dose IV iron treatment strategy over 12 months using the parametric g-formula, restricted to PIVOTAL-like patients. High-dose and low-dose strategies defined by PIVOTAL trialCitation15 protocol as described in ; N=1508 PIVOTAL-like DOPPS patients restricted to emulate PIVOTAL exclusion criteria; Outcomes: (A) all-cause mortality, (B) hemoglobin, (C) serum ferritin, (D) TSAT, (E) ESA dose, (F) IV iron dose.
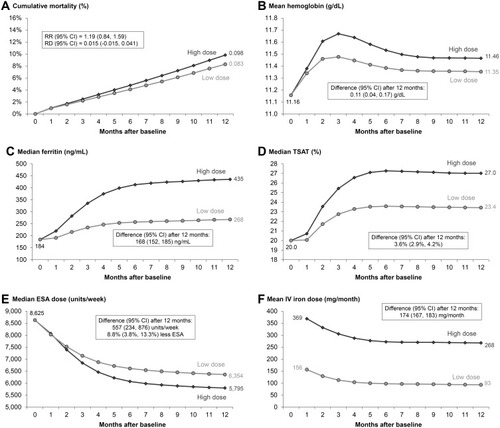
Table 4 Summary of Findings: Comparing PIVOTAL Trial with DOPPS Simulation Using the Full Sample (Objective 1) and PIVOTAL-Like Restricted Subset
