Figures & data
Table 1 STROBE Statement for Cohort Studies with the RECORD Statement Extension—Checklist of Items That Should Be Included in Reports of Observational Studies Using Routinely Collected Health Data
Table 2 Distribution of Users by Study Cohort and Population
Figure 1 Summary results for tacrolimus and pimecrolimus in children and adults combined.
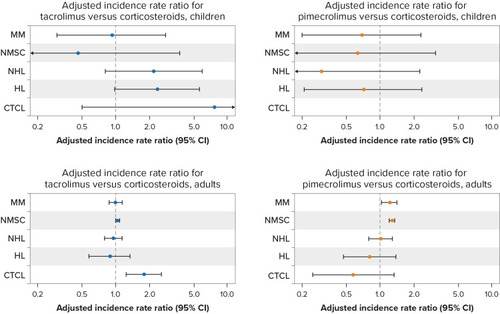
Table 3 Pooled Adjusted Incidence Rate Ratios in Users of Topical Tacrolimus and Topical Pimecrolimus Compared with Users of Topical Corticosteroids—Adults
Table 4 Sensitivity Analysis by Time Since Start of Exposure, by Each Type of Malignancy: Adjusted Incidence Rate Ratios in Users of Topical Tacrolimus Compared with Users of Topical Corticosteroids—Adults
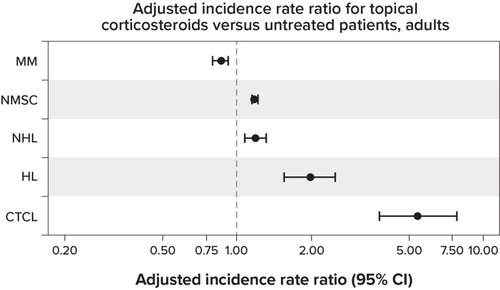
Figure 2 Summary results: untreated adults.