Figures & data
Figure 1 Flowchart for selection of patients for the validation of lines of anti-neoplastic therapy.
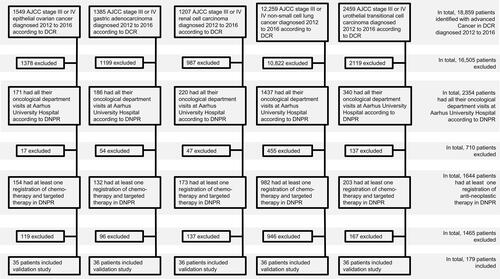
Table 1 Description of the Included Patients with Advanced Epithelial Ovarian Cancer, Gastric Adenocarcinoma, Renal Cell Carcinoma, Urothelial Cell Carcinoma, and Non-Small Cell Lung Cancer Diagnosed in Denmark 2012 to 2016
Figure 2 Percentage agreement between the Time-based algorithm and the Drug-based algorithm, respectively, and the reference standard (medical records) for number of lines of anti-neoplastic therapy for all cancer cohorts combined.
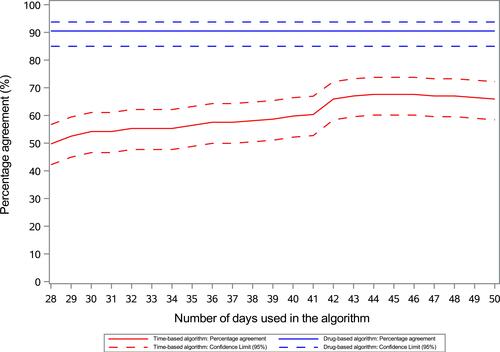
Figure 3 Percentage agreement between the algorithms and the reference standard (medical records) for number of lines of anti-neoplastic therapy for patients with advanced epithelial ovarian cancer (A), gastric adenocarcinoma (B), renal cell carcinoma (C), urothelial cell carcinoma (D), and non-small cell lung cancer (E).
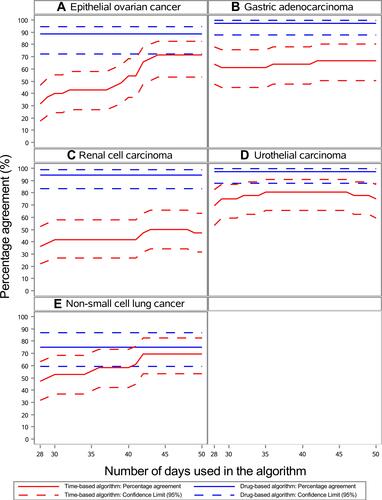
Table 2 Positive Predictive Values for the Number of Anti-Neoplastic Therapy Lines According to Type of Cancer and for All Cohorts Combined for the Time-Based Algorithm 45 Days (a) and the Drug-Based Algorithm 45 Days (b)
Table 3 Positive Predictive Values for the Start Date of the Anti-Neoplastic Therapy Lines, and Duration of Anti-Neoplastic Therapy Lines According to the Time-Based Algorithm 45 Days (a) and the Drug-Based Algorithm 45 Days (b) for All Cohorts Combined
