Figures & data
Figure 1 Identification of study cohort in a flowchart. All patients included in the biobank at the Department of Molecular Medicine, Aarhus University Hospital, Denmark, between January 2012 and December 2018, were identified and included. The cohort was linked by unique 10-digit civil registration number with data from Danish health and medical registries (Danish Colorectal Cancer Group database, the Danish Cancer Registry, the Danish National Registry of Patients, and the Danish Pathology Registry) and used by the algorithm to identify CRC recurrences. A validation cohort was selected for medical chart review to identify recurrences. BIOBANK = CRC biobank at the Department of Molecular Medicine, Aarhus University Hospital, Denmark.
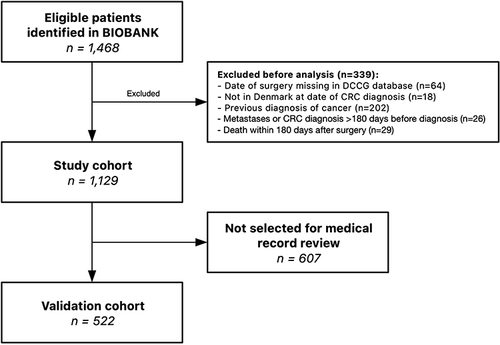
Table 1 Descriptive Characteristics of the Study Cohort and the Validation Cohort
Figure 2 Cumulative incidence curves for CRC recurrence and time to recurrence visualization. (A) Cumulative incidence curves for CRC recurrence within the study cohort. Curves are visualized within each UICC TNM stage treating death as a competing risk. Dashed lines represent lower and upper 95% confidence interval limits. Censoring is illustrated with “|”. No recurrences can be identified by the algorithm within 180 days from CRC diagnosis. No patients were censored within 180 days due to exclusion prior to analysis. (B) Cumulative incidence curves for CRC recurrence within the validation cohort visualized by recurrence identification method. (C) Time to recurrence (TTR) by algorithm vs medical chart review. Datapoints represent the 75 patients with recurrence detected both by the algorithm and through medical chart review. Colored by difference in TTR. The two standard-of-care surveillance CT scans for Danish CRC patients at 12 and 36 months postoperative are annotated. The association was assessed using Spearman correlation. (D) Difference in TTR by algorithm vs medical chart review around postoperative day 180, which is the earliest possible TTR by the algorithm. (E) Difference in TTR by algorithm vs medical chart review at 12 months postoperative. Long dashed and dashed line shows TTR by medical chart review +2 and +4 weeks, respectively. Three recurrences are not registered by the algorithm before +6 weeks from date of recurrence in medical chart (dotted line). (F) Difference in TTR by algorithm vs medical chart review at 36 months postoperative.
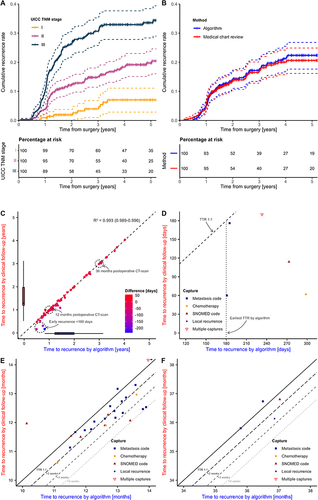
Table 2 Concordance of Recurrences Identified by a Registry-Based Algorithm and Recurrences Identified by Medical Chart Review
