Figures & data
Table 1 Summary Description of Study Cohorts
Figure 1 Study design.
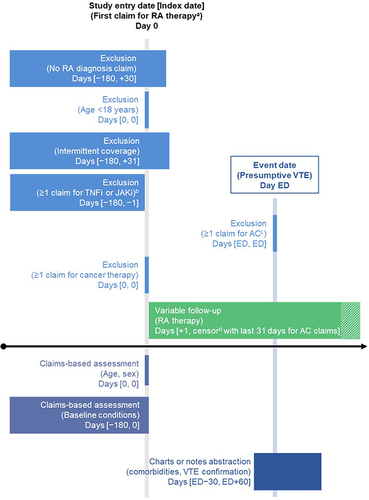
Table 2 VTE Case Definition Criteria
Table 3 Patient Demographic and Clinical Characteristics by Study Sample
Table 4 Performance of VTE Case Definitions
Table 5 Characteristics of Confirmed VTEs
