Figures & data
Figure 1 Comparison of the design of an oncology maintenance therapy trial with a traditional 1L oncology therapy trial. Common 1L oncology clinical trial designs measure OS from initiation of 1L therapy at randomization. Bladder cancer maintenance clinical trial designsCitation4,Citation20 measure OS from initiation of maintenance therapy, a period of time after the initiation of 1L therapy in those patients who had no disease progression after 1L chemotherapy (nonprogressors). Comparing these two trial designs is a controversial issue and has been described in a recent publication.Citation21
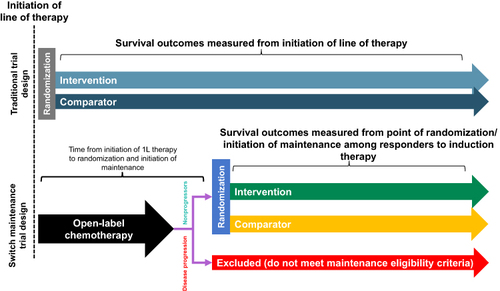
Table 1 Summary of Known and Unknown Data in the 1L PBT–Treated, Maintenance Avelumab–Intended Population of Patients with aUC
Figure 2 Estimated OS in 1L PBT maintenance–intended patients with aUC from initiation of 1L PBT. (A) OS in maintenance eligible, received maintenance from the JAVELIN active arm (green), OS in maintenance eligible, did not receive maintenance from the JAVELIN BSC arm (yellow), and the estimated OS in maintenance ineligible (red) were used to calculate (B) the combined OS in the 1L PBT–treated, maintenance-intended patient population (black) by calculating the weighted average hazard of the three subgroups.Citation4
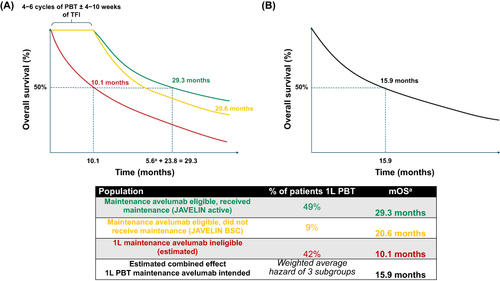
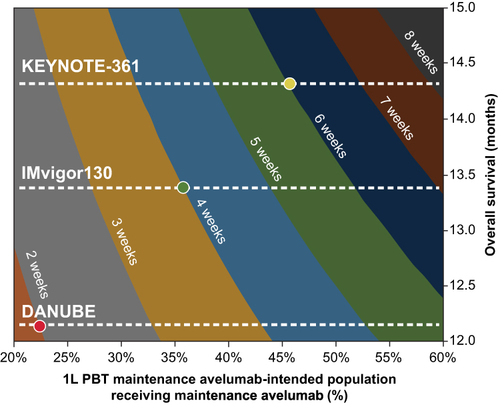
Figure 3 Contour plot of population-level OS impact across included clinical trials.Citation4,Citation13,Citation15,Citation16 By varying the proportion of the 1L PBT maintenance-intended population that received maintenance (x-axis) and varying the mOS associated with PBT between 12.0 and 15.0 months (y-axis), the survival benefit from maintenance compared with PBT alone (based on OS data from the KEYNOTE-361,Citation13 IMvigor130,Citation15 and DANUBECitation17 clinical trials) ranged from 2 to 8 weeks (shown in colored bands). mOS for included clinical trials is shown by the dotted lines. The red dot highlights the expected survival benefit of 2 weeks based on PBT control-arm data from DANUBECitation17 if 22.5% of eligible patients received maintenance avelumab. (The green dot highlights the expected survival benefit of 4 weeks based on PBT control-arm data from IMvigor130Citation15 if 36% of eligible patients received maintA. The yellow dot highlights the expected survival benefit of 6 weeks based on PBT control-arm data from KEYNOTE-361Citation13 if 46% of eligible patients received maintenance (compared with 5 weeks from IMvigor130Citation15 and 4 weeks from DANUBECitation17).