Figures & data
Table 1 Individual IMID-Specific Algorithms Retrieved from the Literature
Figure 1 Flow chart of incident biological drug users included in the study.
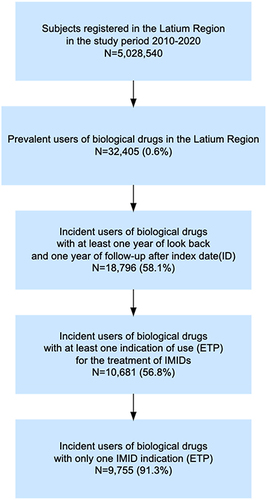
Table 2 Validity Estimates of the META-Algorithm According to the Main Analysis
Figure 2 Percentage of indications of use assigned by the META-algorithm against the reference standard.
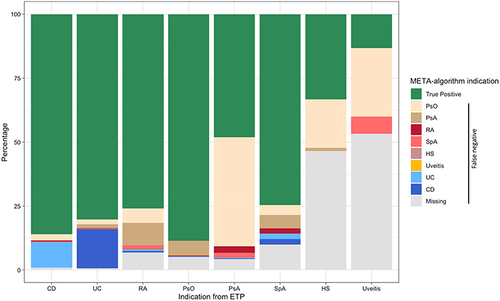
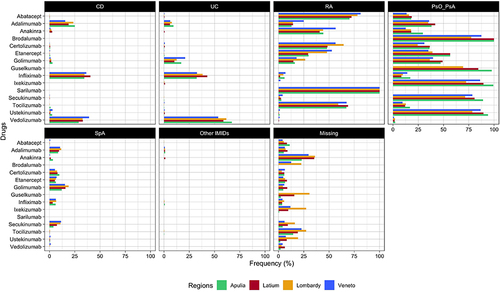
Figure 3 Frequency (%) of indication of use stratified by single biological drug across regions (Apulia, Latium, Lombardy, and Veneto).
Data Sharing Statement
All available data are included in the manuscript and in the Supplementary Materials.
