Figures & data
Table 1 Overview of Data Sources Used for This Study
Table 2 Characteristics of Patients Initially Diagnosed with COVID-19 in the Ambulatory (ie, Outpatient, Emergency Department, or Institutional) Setting Prior to and During COVID-19 Vaccine Availability, by Country
Table 3 90-Day Absolute Risk of Arterial and Venous Thromboembolism Events Among Ambulatory-Diagnosed Patients with COVID-19 Prior to and During COVID-19 Vaccine Availability, by Country
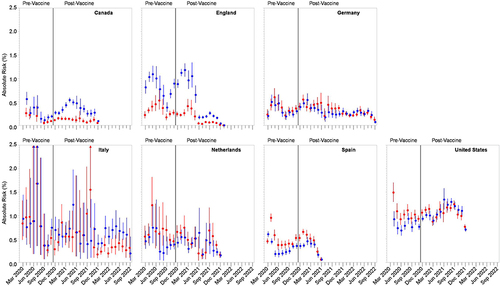
Figure 1 Country-level estimates (95% confidence intervals) of 90-day absolute risk of arterial (red) and venous (blue) thromboembolism events among patients initially diagnosed with COVID-19 in the ambulatory (ie, outpatient, emergency department, or institutional) setting, by month of diagnosis across the pre- and post-vaccine availability periods.