Figures & data
Figure 1 Flowchart Identifying Trials Included in the Study. Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG: The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(6): e1000097. Creative Commons.
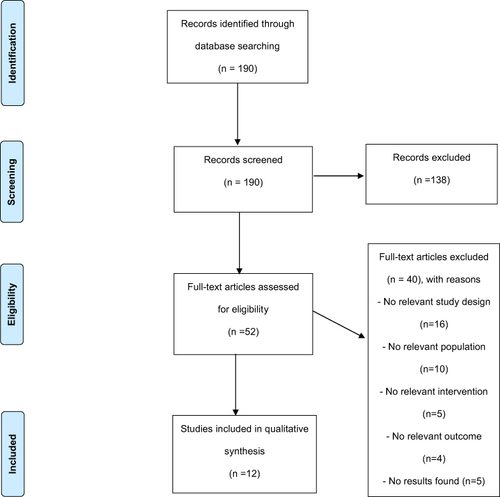
Table 1 Summary of the Current Clinical Trials Evaluating Monoclonal Antibodies (MAB) in COVID-19 Outpatients
Table 2 Summary of the Current Clinical Trials Evaluating Antiviral Treatment in COVID-19 Outpatients
