Figures & data
Table 1 Demographic and clinicopathologic characteristics of 292 patients with gastric cancer
Table 2 Correlation between SII and hematologic parameters
Table 3 Univariate and multivariate Cox regression survival analyses of the SII for the prediction of DFS and OS in patients with gastric cancer
Figure 1 DFS and OS of patients with gastric cancer.
Abbreviations: DFS, disease-free survival; NCT, neoadjuvant chemotherapy; NNCT, non-neoadjuvant chemotherapy; OS, overall survival.
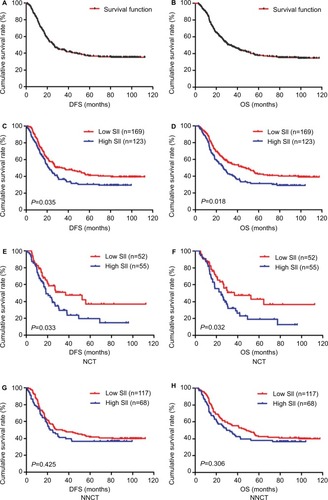
Table 4 1-, 3- and 5-year DFS and OS rates of patients with gastric cancer
Figure 2 The 1-, 3- and 5-year rates of DFS and OS in patients with gastric cancer.
Abbreviations: DFS, disease-free survival; NCT, neoadjuvant chemotherapy; NNCT, non-neoadjuvant chemotherapy; OS, overall survival.
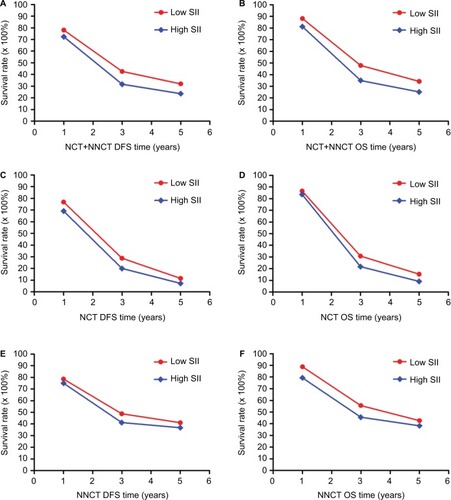
Figure 3 DFS and OS for the SII of patients with gastric cancer in pathologic stage.
Abbreviations: DFS, disease-free survival; OS, overall survival.
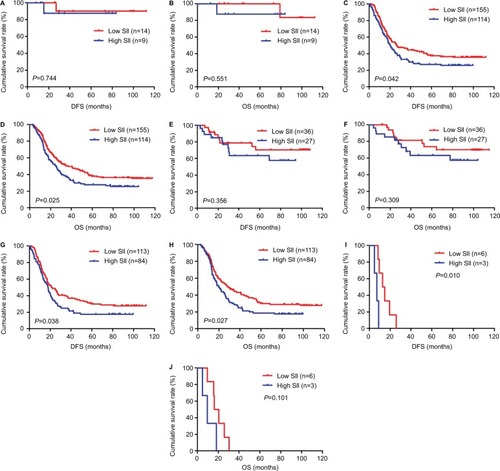
Table 5 Correlation between SII and neoadjuvant chemotherapy or postoperative chemotherapy of patients with gastric cancer
Figure 4 DFS and OS of patients with gastric cancer receiving neoadjuvant chemotherapy and postoperative chemotherapy in the NCT group.
Abbreviations: DFS, disease-free survival; NCT, neoadjuvant chemotherapy; OS, overall survival.
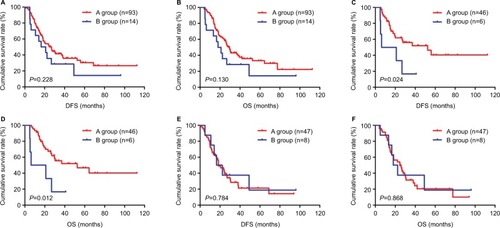
Figure 5 DFS and OS of patients with gastric cancer receiving postoperative chemotherapy in the NNCT group.
Abbreviations: DFS, disease-free survival; NNCT, non-neoadjuvant chemotherapy; OS, overall survival.
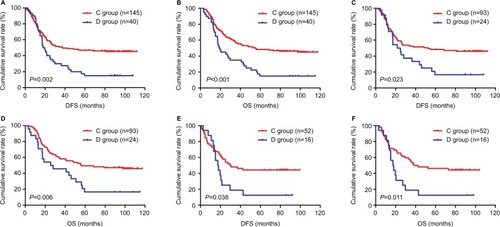
Table 6 Main toxicities according to NCI-CTC scale of the patients with gastric cancer undergoing neoadjuvant chemotherapy
