Figures & data
Figure 1 Fatal toxic epidermal necrolysis after cetuximab treatment for 8 weeks.
Notes: A 74-year-old man who had moderately differentiated metastatic colon adenocarcinoma presented diffuse erythematous plaques with dusky red centers on trunk and extremities after treatment with cetuximab for 8 weeks. The skin rashes were confluent and formed large blisters or skin detachments involving more than 70% of the body surface area.
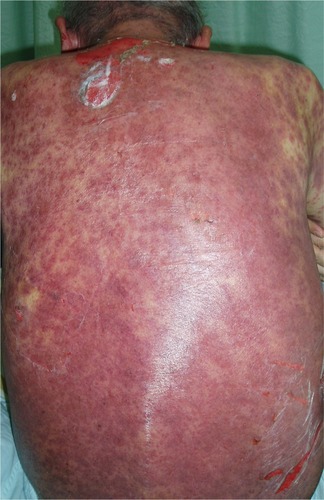
Figure 2 Drug rash with eosinophilia and systemic symptoms after erlotinib treatment for 4 weeks.
Notes: A 60-year-old woman with EGFR-mutant metastatic lung adenocarcinoma treated with erlotinib for 4 weeks. She developed generalized infiltrative exanthema on trunk and limbs accompanied by fever, acute liver failure, coagulopathy, and leukocytosis with eosinophilia. Further lymphocyte activation testing confirmed a hypersensitivity reaction to erlotinib.
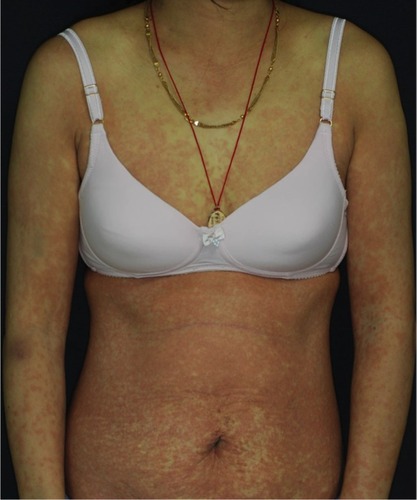
Table 1 Targeted anticancer therapies and immunotherapies-induced severe cutaneous adverse reactions (n=73)
Table 2 Tolerability follow-up for rechallenge or alternatives in patients with targeted anticancer therapies and immunotherapyinduced severe cutaneous adverse reactions (n=25)
Table 3 Mortality in severe cutaneous adverse reactions related to targeted anticancer therapies and immunotherapies (n=14)
Table 4 Targeted anticancer therapies and immunotherapy-induced severe cutaneous adverse reaction cases with multiple concomitant medication (n=24)
