Figures & data
Table 1 Clinicopathological characteristics of patients with stage III colon cancer
Figure 1 Median cycle of XELOX adjuvant chemotherapy received by patients for each tumor location.
Notes: *XELOX adjuvant chemotherapy was administered as follows: oxaliplatin at a dose of 130 mg/m2 was administered intravenously on day 1, and capecitabine was administered orally at a dosage of 1000 mg/m2 twice daily on days 1–14 for a 3-week cycle.
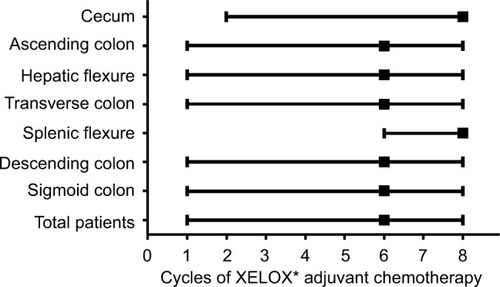
Table 2 Treatment-related toxicities during adjuvant chemotherapy in patients with stage III colon cancer after curative resection
Table 3 Postoperative metastatic patterns of patients with stage III colon cancer after curative treatment
Table 4 Univariate and multivariate analyses of prognostic factors for overall survival in patients with stage III colon cancer who received curative treatment
Figure 2 Kaplan–Meier curves of patients with stage III colon cancer grouped by right- and left-sided colon and stratified by stage.
Notes: (A) Disease-free survival (DFS) of all patients. (B) Overall survival (OS) of all patients. (C) DFS of patients with stage IIIA–B colon cancer. (D) OS of patients with stage IIIA–B colon cancer. (E) DFS of patients with stage IIIC colon cancer. (F) OS of patients with stage IIIC colon cancer.
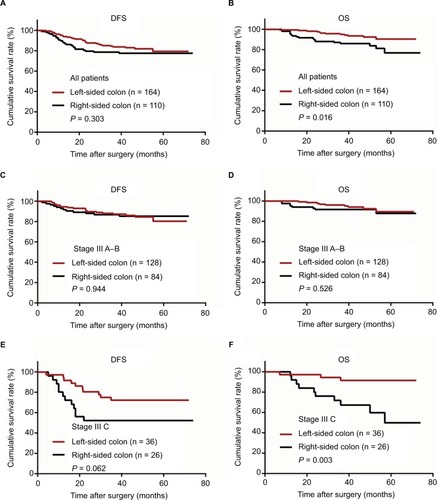
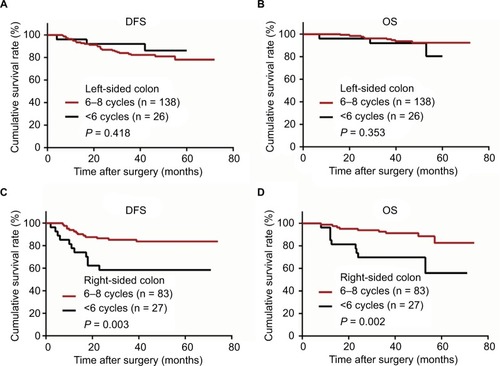
Figure 3 Kaplan–Meier curves of patients with stage III colon cancer in right- and left-sided colon stratified by cycles of XELOX* adjuvant chemotherapy.
Notes: (A) Disease-free survival (DFS) in left-sided colon cancer. (B) Overall survival (OS) in left-sided colon cancer. (C) DFS in right-sided colon cancer. (D) OS in right-sided colon cancer. *XELOX adjuvant chemotherapy was administered as follows: oxaliplatin at a dose of 130 mg/m2 was administered intravenously on day 1, and capecitabine was administered orally at a dosage of 1000 mg/m2 twice daily on days 1–14 for a 3-week cycle.