Figures & data
Figure 1 CD44 is dysregulated in cancers and upregulated in hepatobiliary tumors.
Notes: (A) CD44 mRNA expression level in TCGA data analysis. CD44 mRNA was upregulated in tumor tissues of LIHC and CHOL. (B) Panoramic scanning for GBC TMA. (C) Representative CD44 staining in GBC tissues and normal samples. (D) Histological scoring of CD44 in GBC tissues and normal samples; CD44 was overexpressed in GBC tissues compared with the non-tumor tissues (P=0.0036). (E) Related CD44 mRNA expression in GBC tissues compared with paired non-tumor tissues (P<0.0001). *P<0.05.
Abbreviations: CD44, cluster of differentiation 44; TCGA, The Cancer Genome Atlas; LIHC, liver hepatocellular carcinoma; CHOL, cholangiocarcinoma; GBC, gallbladder cancer; TMA, tissue microarray; BLCA, bladder urothelial carcinoma; BRCA, breast cancer; COAD, colon cancer; ESCA, esophagus cancer; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, renal cancer; KIRP, kidney renal papillary cell carcinoma; LUAD, lung cancer; LUSC, lung squamous cell carcinoma; PRAD, prostate adenocarcinoma; READ, rectal cancer; STAD, stomach cancer; THCA, thyroid cancer; UCEC, uterine corpus endometrial carcinoma; ACC, adrenocortical carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B-cell lymphoma; LAML, acute myeloid leukemia; LGG, brain lower drade glioma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; SARC, sarcoma; SKCM, skin cutaneous melanoma; TGCT, testicular germ cell tumors; THYM, thymoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma.
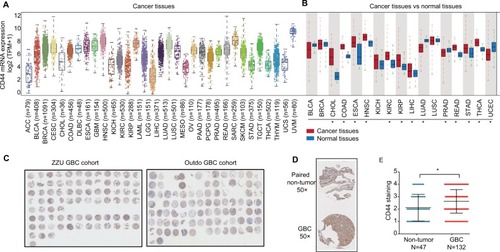
Figure 2 CD44 is overexpressed in GBC tissues and negatively correlated with OS.
Notes: (A) Representative CD44 and immunohistochemical staining patterns with different staining scores in GBC tissues. (B) Histological scoring of CD44 according to TNM classification. CD44 expression was notably high in GBC patients with advanced TNM stage (*P<0.05, **P<0.01). (C) Kaplan–Meier survival analysis between expression of CD44 (red, high CD44 expression; green, low CD44 expression). GBC patients with high CD44 expression had markedly shorter OS than those with low CD44 expression (P=0.0214).
Abbreviations: CD44, cluster of differentiation 44; GBC, gallbladder cancer; H&E, hematoxylin-eosin.
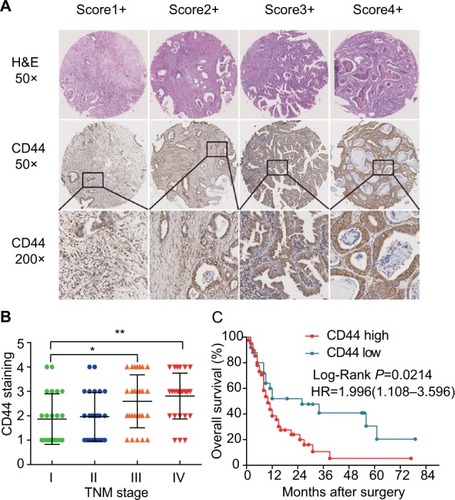
Table 1 The relationship between CD44 expression and clinicopathological features of gallbladder cancer
Table 2 Univariate and multivariate analyses of OS of gallbladder carcinoma
Figure 3 RNAi-mediated CD44 silencing inhibits in vitro GBC cells’ proliferation, migration and invasion.
Notes: (A) Dose-dependent CD44-siRNA downregulated the expression of CD44. (B) CCK-8 assay showed that CD44 silencing attenuated proliferation of GBC-SD and NOZ cells. (C) EDU assay confirmed that CD44 knockdown suppresses proliferation of GBC-SD and NOZ cells. (D) CD44 silencing caused a remarkable suppression of cell migration in GBC-SD and NOZ cells using wound-healing assay. (E) The invasiveness of GBC-SD and NOZ cells infected with CD44-siRNA was significantly suppressed according to cell invasion assay. **P<0.01.
Abbreviations: RNAi, RNA interference; CD44, cluster of differentiation 44; GBC, gallbladder cancer; NC, nonspecific control siRNA.
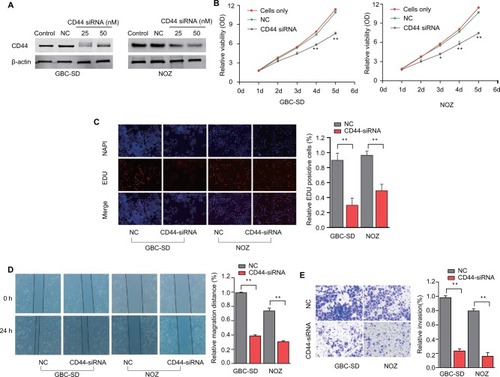
Figure 4 CD44 downregulation suppresses colony formation ability of CSCs.
Notes: (A) The colony formation ability of CSCs treated with CD44-siRNA was decreased. (B) Formation of spheres from GBC cells transfected with CD44-siRNA accessed by three-dimensional cell culture. The diameter of cancer spheroids formed from GBC-SD cells with CD44 silencing was significantly smaller than the control group. All the results were reproducible in three independent experiments. **P<0.01.
Abbreviations: CD44, cluster of differentiation 44; CSCs, cancer stem cells; GBC, gallbladder cancer; NC, nonspecific control siRNA.
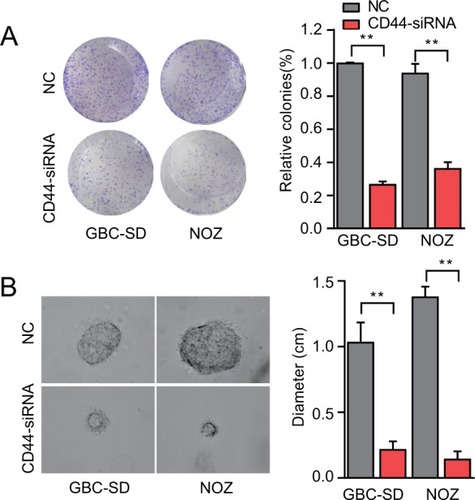
Table S1 Cell lines used in this study
Table S2 Information on antibodies used in this study
Table S3 siRNA sequence used in this study
Table S4 qRT-PCR primer sequence used in this study
