Figures & data
Figure 1 AFP overexpression and derived supernatant enhanced proliferation, invasion, and migration in established APGC cells.
Notes: (A and B) AFP overexpression in GC cells (HGC27 and AGS) was confirmed by PCR and ELISA with HepG2 cells as the positive control. (C) AFP-secretion levels of AFP-overexpressing GC cells compared to their controls were dynamically monitored by ELISA. (D and E) Impacts of AFP overexpression and derived supernatant on cell proliferation, invasion, and migration abilities in GC cells were measured by CCK8 and transwell assays, respectively. *P<0.05 by ANOVA. Data expressed as mean ± SD.
Abbreviation: APGC, AFP-producing gastric cancer.
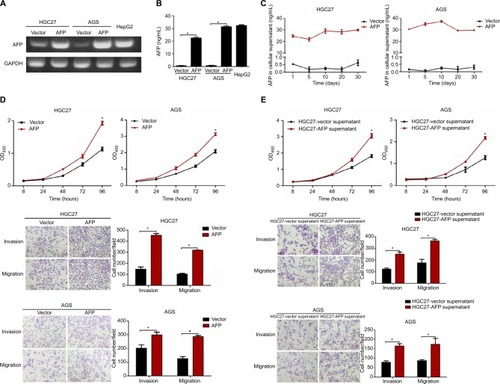
Figure 2 AFP promoted growth and metastasis in established APGC xenografts.
Notes: HGC27-AFP and HGC27-vector cells were subcutaneously injected into NOD/SCID mice (n=5/group). (A) Tumor volume was measured every 3 days after 2 weeks; tumor-growth curves shown. Data expressed as mean ± SD. *P<0.05 by repeated-measure ANOVA. (B) FFPE sections were stained with Ki67 for IHC analysis. NOD/SCID mice inoculated with HGC27-AFP and HGC27-vector cells by IP route and IOCV (n=5/group). (C and D) Representative images of ascites and peritoneal dissemination in IP groups and lung metastasis in IOCV groups. Black arrows, disseminated tumor foci. (E) Comparisons of ascites, peritoneal dissemination, and distant metastasis capacity between mice bearing HGC27-AFP and HGC27-vector cells. (F) Representative H&E-stained foci of peritoneal spreading in liver and spleen from IP groups and of distant metastasis in lung from IOCV groups. Red arrows, tumor foci.
Abbreviations: APGC, AFP-producing gastric cancer; FFPE, formalin-fixed, paraffin-embedded; IHC, immunohistochemistry; IOCV, injection of caudal vein; IP, intraperitoneal injection; NOD, nonobese diabetic; SC, subcutaneous; SCID, severe combined immunodeficiency.
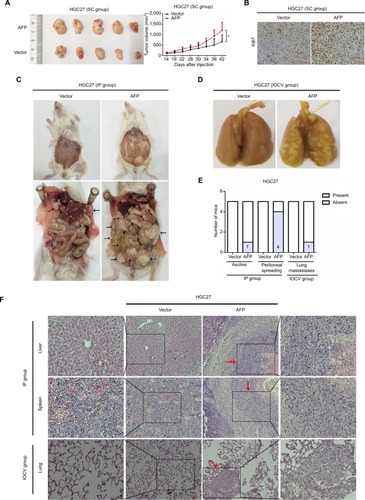
Figure 3 AFP-activated canonical Wnt signaling in established APGC preclinical models.
Notes: (A) The top 20 KEGG-identified candidate pathways enriched by a comparison of AFP-overexpressing HGC27 cells and the control. (B–D) Cellular, supernatant, and tissue proteins extracted from indicated groups were probed against a variety of Wnt-signaling-associated antibodies as indicated. (E) TOPflash and FOPflash plasmids were cotransfected with Renilla plasmids and subjected to dual-luciferase assays after 48 hours in AFP-overexpressing HGC27 and AGS cells and their controls. Reporter activity was normalized to Renilla luciferase activity. Data expressed as mean ± SD. *P<0.05 by ANOVA.
Abbreviations: APGC, AFP-producing gastric cancer; KEGG, Kyoto Encyclopedia of Genes and Genomes; Padj, adjusted P-value.
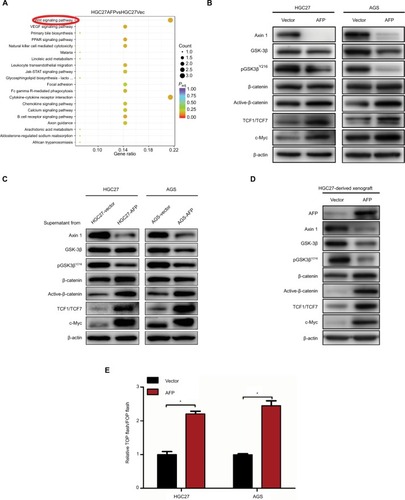
Figure 4 Axin 1 overexpression reduced AFP-mediated Wnt-pathway activation and malignancy in established APGC cells.
Notes: (A–D) After Axin 1 knockdown using siRNAs in GC cells and (E–H) Axin 1 overexpression in AFP-overexpressing GC cells for 48 hours, Wnt-signaling-involved protein-expression levels, β-catenin-mediated TCF transcriptional activity, and cell-proliferation, -invasion, and -migration abilities were determined by immunoblotting, dual-luciferase, CCK8, and transwell assays, respectively. Data expressed as mean ± SD. *P<0.05 by ANOVA.
Abbreviation: APGC, AFP-producing gastric cancer.
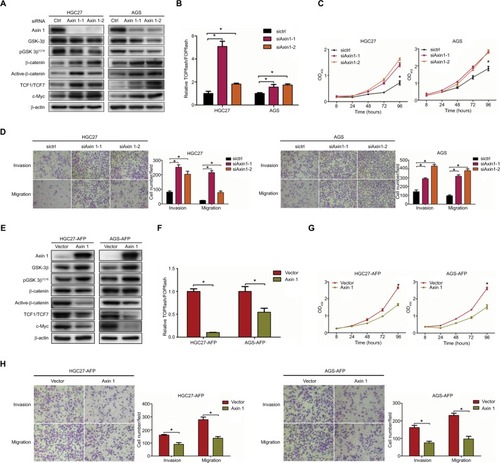
Figure 5 Wnt-pathway inhibitor reduced AFP-mediated Wnt-pathway activation and malignancy in established APGC cells.
Notes: AFP-overexpressing GC cells and their controls were treated in the absence or presence of Wnt-pathway inhibitor XAV939 (50 µM) for 48 hours. (A) Immunoblotting was carried out for Wnt axis-associated proteins and (B and C) proliferation and invasion/migration abilities were measured by CCK8 and Transwell assays with/without Matrigel, respectively. Data expressed as mean ± SD. *P<0.05 by ANOVA.
Abbreviation: APGC, AFP-producing gastric cancer.
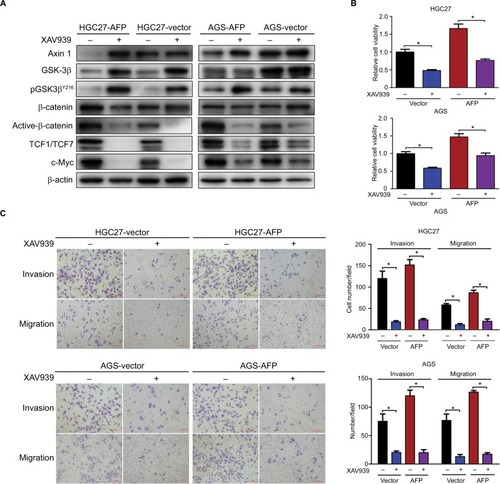
Figure 6 Representation of targeting Wnt pathways responsible for AFP-associated malignancy in APGC.
Notes: Compared to the control, AFP overexpression promoted AFP-supernatant secretion, which activated canonical Wnt signaling (marked by inactive destruction complex including GSK3β dephosphorylation/inactivation, and reduced Axin 1, increased active β-catenin, augmented TCF transcriptional activity and upregulated target gene c-MYC) to enhance growth and metastasis in GC. Wnt-pathway blockade by Axin 1 overexpression or small-molecule inhibitor XAV939 (Axin1 stabilizer) impeded AFP-mediated malignancy in AFP-overexpressing GC, indicating therapeutic potentials of targeting Wnt pathways against APGC.
Abbreviation: APGC, AFP-producing gastric cancer.
Figure S2 AFP overexpression had no effects on PI3K/Akt signaling or VEGF expression in gastric cancer cells.
Notes: Proteins extracted from AFP-overexpressing gastric cancer cells and their controls were probed against indicated antibodies.
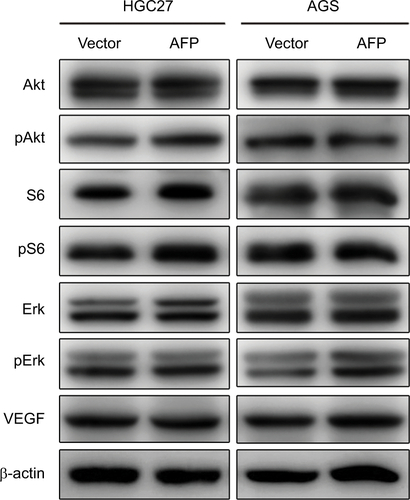
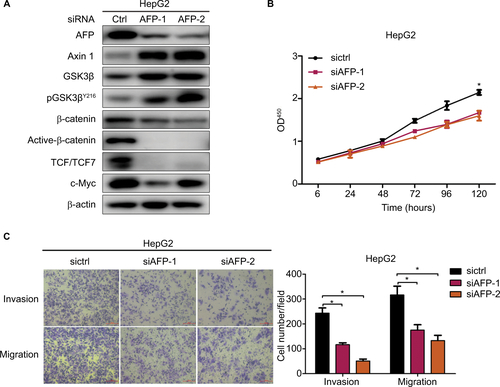
Figure S3 AFP knockdown inactivated the Wnt cascade and inhibited growth, invasion, and migration in HepG2 cells.
Notes: Proteins were probed against indicated antibodies involving Wnt signaling, and cell growth, invasion, and migration were compared in AFP-knockdown HepG2 cells and controls. Data expressed as mean ± SD. *P<0.05 by ANOVA analysis.