Figures & data
Figure 1 TGF-β regulation of cancer tissue.
Notes: Enhanced TGF-β secretion by cancer and stromal cells promotes the development of a dense myofibroblastic microenvironment with CAFs expressing α-smooth muscle actin, together with immune tolerance and EMT, thus plagiarizing the features of the placental trophoblast-decidua interface. Conversely, inhibition of cancer cell growth by the physiological TGF-β cytostatic program is virtually nonexistent, due to various obstacles: impairment of the TGF-β/SMAD axis and of the SMAD-driven gene transcription, high expression of SMAD7, and actions of autocrine growth factors and β-hCG. The β-chain of hCG, a hormone normally produced by trophoblast, may interfere with TGF-β receptors, thus preventing the normal action of TGF-β. The above figure is valid for trophoblastic cells (in place of cancer cells), with the SMAD cytostatic program being silenced by RTK ligands and β-hCG, and for decidual myofibroblasts (in place of CAFs).
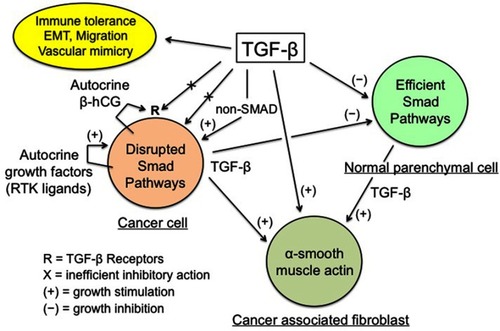
Figure 2 Apparent conflicting actions of the immune system on cancer cells.
Notes: Cancer tissue conveniently takes advantage of the physiological, immune-related healing mechanisms and improves its growth potential this way. Moreover, cancer cells induce a trophoblastic-like immune tolerance limiting the immune destructive action against them. aFor example, wounds or invasion of endometrium by trophoblast. bLimited by trophoblastic-like immune tolerance.
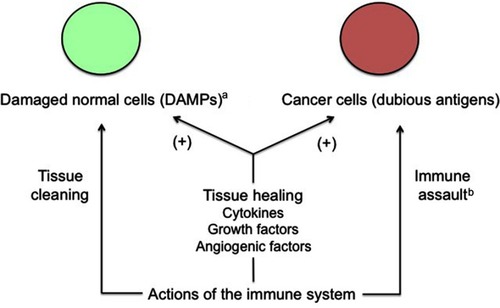
Figure 3 Simplified representation of the signaling maze of TLRs, TNF-α and NF-κB.
Notes: NF-κB is a pleiotropic transcription factor with ambivalent, pro- and antitumor actions. PAMPs and DAMPs activate NF-κB via TLRs. TNF-α is an inflammatory cytokine present both downstream and upstream of NF-κB. TGF-β activates NF-κB via the interlinked SMAD and MAPK/Akt signaling cascades, and NF-κB induces a SMAD7-driven negative feedback loop. All these facts clearly mean that the final action of the represented system, that works, among others, in cancer and trophoblast, is not unequivocal and that it strongly depends on a lot of interfering factors. Striking similarities exist between cancer and trophoblast as regards the immune-related fighting, cleaning, and healing actions.
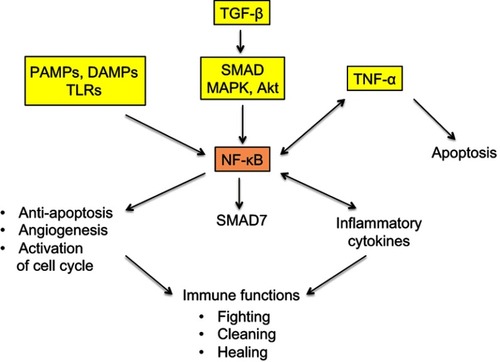
Figure 4 Expression of the trophoblastic Trop-2 biomarker in cancer cells.
Notes: Both overexpression and, in a few cases, nonexpression are observed in carcinogenesis. Overexpression of Trop-2 is assumed to result from the putative trophoblastic-like transdifferentiation and subsequent activation of the TACSTD2 gene. Nonexpression simply means that the TACSTD2 gene is defective or epigenetically silenced, while the other trophoblastic biomarkers should logically be expressed. Trop-2 typically promotes and less frequently restricts cancer progression, depending on the histotype.
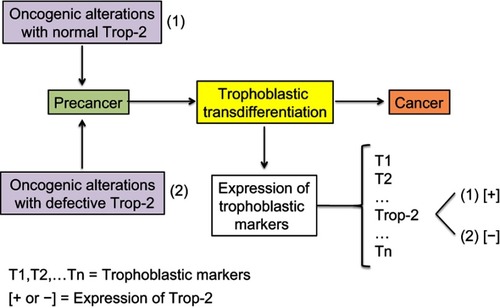
Figure 5 Model of cancer tissue development.
Notes: The general pattern and hierarchy of tissue structure, based on stem cells (SC) with self-renewal capabilities and differentiated daughter cells (DC) with decreased proliferation potential, is supposed to be the same for normal, mutated, or malignant cells. Stem cells are rare but certain of them have a metastatic potential and those being in a quiescent state may explain posttreatment relapses. They are hence key actors of the malignant process. Differentiated cells form the bulk of the tumor, but cannot create metastases nor induce posttreatment relapses because their lifetime is limited. The probability of trophoblastic-like transdifferentiation, that provides certain precancer cells with the vital logistical support leading to malignancy, is infinitesimal. More precisely, the transdifferentiation component that interests us here is essentially that which drives the extravillous phenotype.
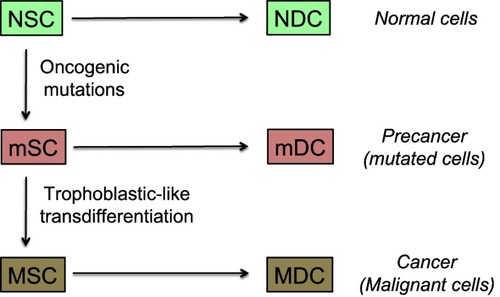
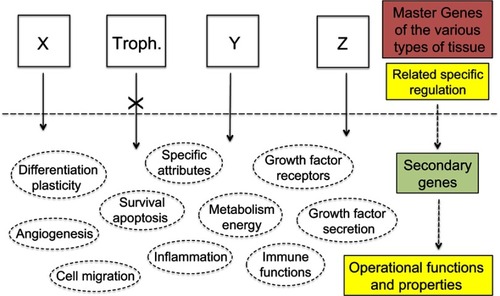
Figure 6 Therapeutic strategies.
Notes: The current anti-cancer therapies target operational functions and properties common to all cells and thus have adverse side effects against normal tissues for which use of certain of these functions and properties is potentially vital. Exclusively targeting the specific products of the trophoblastic master genes would de facto make cancer cells lack a suitable logistical support to survive whereas normal tissues would not be impacted as every operational function and property of normal cells would remain unaltered. X, Y, Z represent the master genes that determine the various types of tissue, eg, X may be the group of epidermis master genes, alike Y for kidney epithelium, or Z for thyroid epithelium, etc. “Troph.” represents the trophoblastic master genes.