Figures & data
Figure 1 Baohuoside I inhibit hTERT-HPNE, PANC-1 and CFPAC-1 cells proliferation. CCK8 assay following hTERT-HPNE (A), PANC-1 (B) and CFPAC-1 (C) cells incubated with 10μM, 20μM, 30μM, 40μM, 50μM, 60μM, 70μM, 80μM, 90μM Baohuoside I or an equal volume of DMEM medium for 24h. Label-free Real-time Cellular Analysis (RTCA) following PANC-1 (D) and CFPAC-1 (E) cells incubated with Baohuoside I (20μM, 50μM) or an equal volume of DMEM medium for 24h. (F) Colony formation assay following PANC-1 and CFPAC-1 cells incubated with Baohuoside I (20μM, 50μM) or an equal volume of DMEM medium for 24h. The pancreatic cancer cells' viability rate in Baohuoside I-treated cells was significantly decreased compared with hTERT-HPNE cells. Baohuoside I significantly inhibited proliferation in pancreatic cancer, and the inhibition is concentration dependent. Data are presented as mean ± SD, N = 3; **P<0.01; ***P<0.001; ****P<0.0001, compared with control.
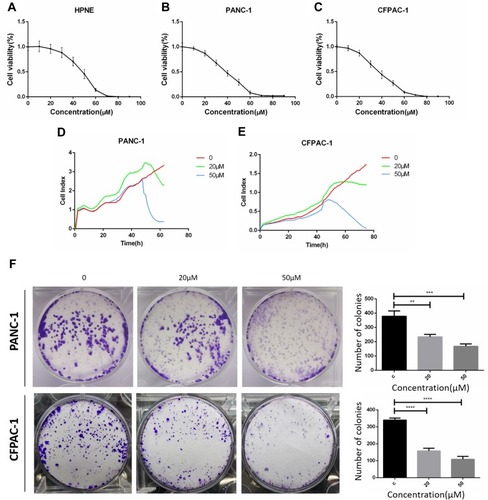
Figure 2 Baohuoside I inhibit PANC-1 and CFPAC-1 invasion and migration. (A) Transwell assay following PANC-1 and CFPAC-1 cells incubated with Baohuoside I (20μM, 50μM) or an equal volume of DMEM medium for 24h. (B) Wound healing assay following PANC-1 and CFPAC-1 cells incubated with Baohuoside I (20μM, 50μM) or an equal volume of DMEM medium for 24h. Baohuoside I significantly inhibited invasion and migration in PANC-1 and CFPAC-1 cells of pancreatic cancer. And the invasion and migration inhibited by Baohuoside I is concentration dependent. Data are presented as mean ± SD, N = 3; ***P<0.001; ****P<0.0001, compared with control.
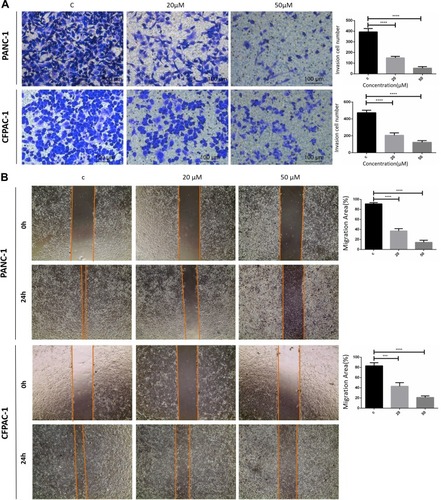
Figure 3 Baohuoside I promote PANC-1 and CFPAC-1 apoptosis. Flow cytometry for apoptosis [apoptosis ratio was calculated as (Q2+Q3)/(Q1+Q2+Q3+Q4)] of PANC-1 and CFPAC-1 cells incubated with Baohuoside I (20μM, 50μM) or an equal volume of DMEM medium for 24h. Baohuoside I significantly promoted apoptosis in PANC-1 and CFPAC-1 cells of pancreatic cancer. And the apoptosis induced by PANC-1 and CFPAC-1 is concentration dependent. Data are presented as mean ± SD, N = 3; ***P<0.001; ****P<0.0001, compared with control.
![Figure 3 Baohuoside I promote PANC-1 and CFPAC-1 apoptosis. Flow cytometry for apoptosis [apoptosis ratio was calculated as (Q2+Q3)/(Q1+Q2+Q3+Q4)] of PANC-1 and CFPAC-1 cells incubated with Baohuoside I (20μM, 50μM) or an equal volume of DMEM medium for 24h. Baohuoside I significantly promoted apoptosis in PANC-1 and CFPAC-1 cells of pancreatic cancer. And the apoptosis induced by PANC-1 and CFPAC-1 is concentration dependent. Data are presented as mean ± SD, N = 3; ***P<0.001; ****P<0.0001, compared with control.](/cms/asset/f48250e5-c3a8-4c4c-a92a-bac87eb3ecdf/dcmr_a_12186857_f0003_c.jpg)
Figure 4 Baohuoside I reduces glycolysis in pancreatic cancer cells. (A and B) Overall ECAR curve of PANC-1and CFPAC-1 cells treated with or without Baohuoside I (0, 20, 50 mM) for 24 h. Injection order: glucose (10mM), oligomycin (1 μM), and 2-DG (50 mM) for both PANC-1 and CFPAC-1 cells. (C) Basal glycolysis. (D) Maximal glycolysis. (E) Glycolysis capacity. (F) Glycolysis metabolism. Data are presented as the mean ±SD (ns, not significant, **P < 0.01; ***P<0.001; ****P<0.0001). The representative images represent the mean ECAR ± SD of eight replicates.
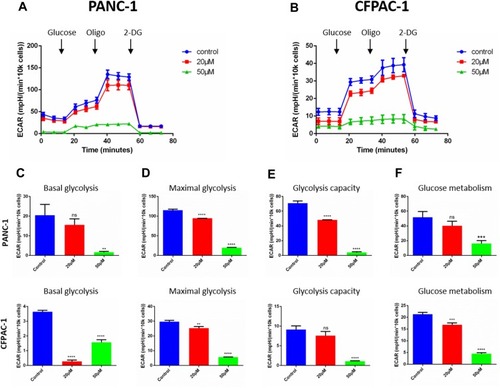
Figure 5 Baohuoside I reduces mitochondrial respiration in pancreatic cancer cells. (A and B) Overall oxygen consumption rate (OCR) curve of PANC-1 and CFPAC-1 cells treated with or without Baohuoside I (0, 20, 50μM) for 24 h with the Seahorse XF96 Extracellular Flux Analyzer. Injection order: oligomycin (2.5 μM), FCCP (2 μM), and rotenone (0.25 μM), and antimycin A (0.25 μM) for both PANC-1 and CFPAC-1 cells. The overall OCR curves were plotted as the mean OCR ± SD of eight replicates. (C) Basal respiration, (D) maximal respiration, and (E) ATP production were assessed, respectively. Data are presented as the mean ± SD (ns, not significant, *P < 0.05; ***P<0.001; ****P<0.0001).
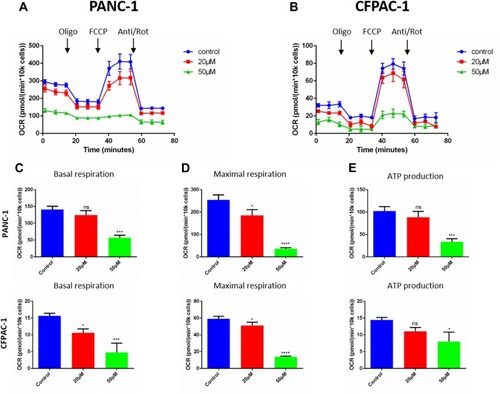
Figure 6 Baohuoside I inducing apoptosis is dependent on mTOR/S6K1-Caspases/Bcl2/Bax apoptotic signaling. After 24 h treatment with Baohuoside I (20μM, 50μM), (A and B) the expression of AMPK/mTOR signaling pathway autophagy and apoptosis‐related proteins was detected by Western blotting analysis. (C) Mechanism of Action of Baohuoside I on pancreatic cancer cells.
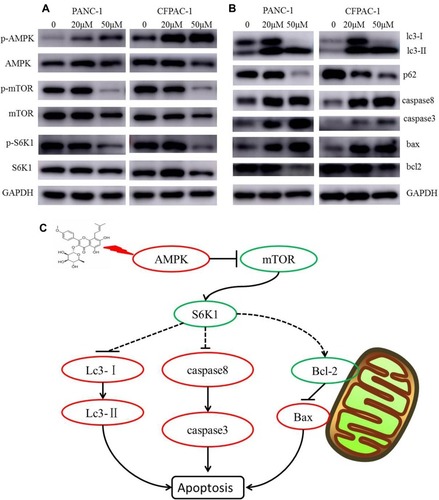
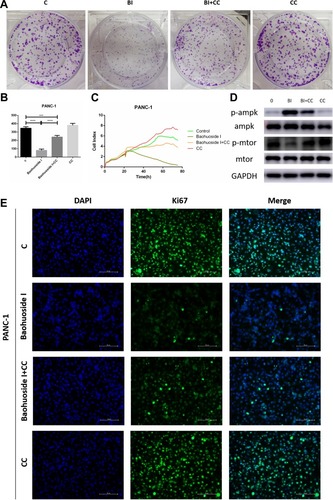
Figure 7 Compound C reversed Baohuoside I induced inhibition of proliferation of pancreatic cancer cells through AMPK suppression. (A and B) The colony formation assays showed that Compound C (10μM) restored the inhibitory effect of Baohuoside I (50μM) on PANC-1 cells proliferation. (C) The RTCA showed that the Baohuoside I induced suppressing effect of proliferation was reversed by Compound C. (D) the pretreatment of Compound C down-regulated the expression of p-AMPK and up-regulated the expression of the p-mTOR in PANC-1 cells treated with Baohuoside I. (E) Compound C was found to attenuate the suppression expression of the cell proliferation marker Ki-67 induced by Baohuoside I in PANC-1 cells. Data are presented as the mean ± SD (***P < 0.001; ****P<0.0001).
Abbreviations: BI, Baohuoside I; C, Control; CC, Compound C.