Figures & data
Figure 1 GSDMB is different from other gasdermins in its structure and domain architecture. The protein of GSDMA (A), GSDMB (B), GSDMC (C), GSDMD (D) and GSDME (E) is shown as cartoon. Blue cartoon represents the helices of protein. Red cartoon represents the sheets of protein. Yellow cartoon represents the coils of protein.
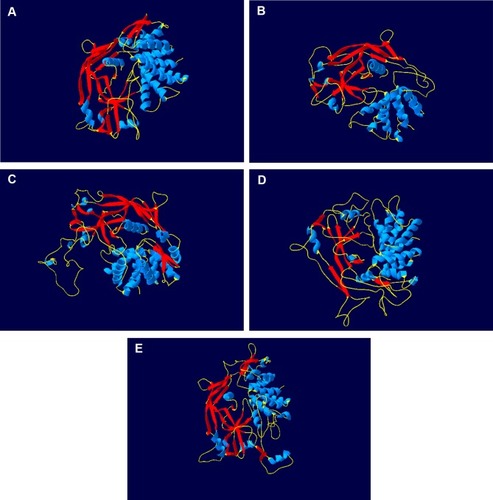
Figure 2 Cleavage of GSDMB protein by caspase-1 induces pyroptosis. Caspase-1 promotes the cleavage of GSDMB and the releasing of the N-terminal effector domain and the C-terminal inhibitory domain. The N-terminal domain oligomerizes in the cell membrane and forms pores, which leads to pyroptosis.
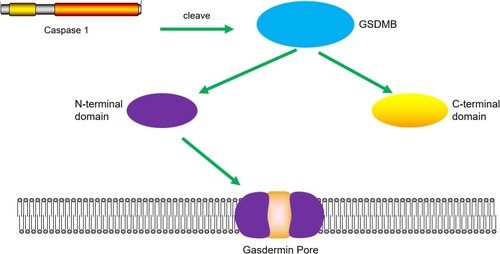
Figure 3 GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. The binding of GSDMB with the CARD domain of caspase-4 result in the oligomerization of the caspase-4 proteins, and caspase-4 promotes the cleavage of GSDMD and the releasing of the N-terminal effector domain and the C-terminal inhibitory domain. The N-terminal domain oligomerizes in the cell membrane and forms pores, which leads to pyroptosis.
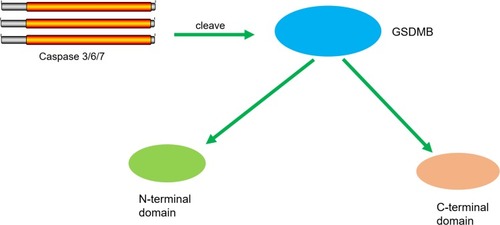
Figure 4 GSDMB could be cleaved by caspase-3/-6/-7. Caspase-3/-6/-7 promotes the cleavage of GSDMB and the releasing of the N-terminal effector domain and the C-terminal inhibitory domain.
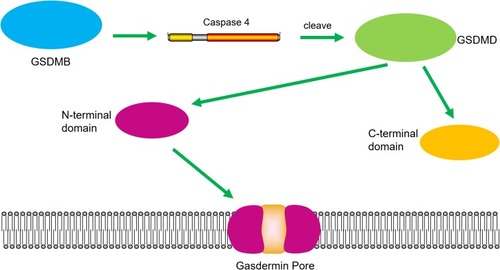
Table 1 Differences in the Experiments About the Mechanism of GSDMB in Pyroptosis
Table 2 GSDMB in Different Cancers
