Figures & data
Figure 1 Histologic features of AFPGC and HAS. (A) HE staining of primary lesion of HAS. (B) HE staining of metastatic lesion of the same HAS patient as (A). (C) HE staining of AFPGC sample without typical HAS. (D) Immunohistochemical staining for AFP (negative) of AFPGC sample without typical HAS. (E) PD-L1 positive sample by immunohistochemical staining with SP263. (F) PD-L1 negative sample by immunohistochemical staining with SP263.
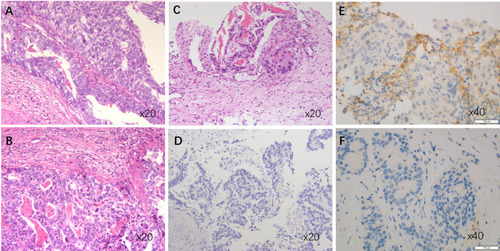
Figure 2 The distribution of AFPGC and HAS in enrolled patients. Nineteen patients were with AFP positive and 13 patients were diagnosed as HAS. Eleven HAS patients had high serum AFP.
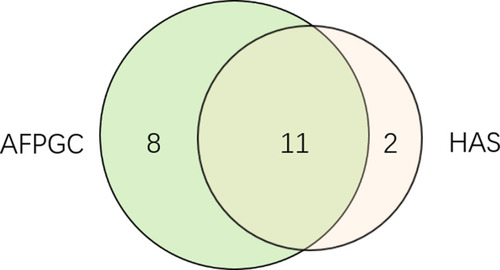
Table 1 Baseline Characteristics of Patients
Table 2 Comparison of Objective Response Rates to Different Treatment Regimens
Figure 3 Comparison of progress-free survival (A) and overall survival (B) of AFPGC/HAS patients treated with chemotherapy and those receiving immunotherapy plus chemotherapy.
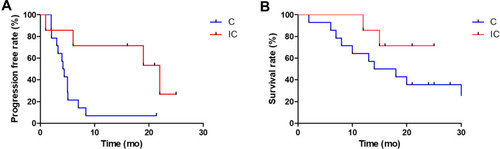
Table 3 The Treatment of Immune Therapy Group and Evaluation of Effectiveness
