Figures & data
Table 1 Clinicopathological Characteristics of 136 Patients Enrolled
Table 2 Univariate Analysis of Clinicopathological Factors Associated with RFS
Table 3 Multivariate Cox Proportional Hazards Model of RFS
Figure 2 Kaplan–Meier Curves of RFS based on chemotherapy regime.
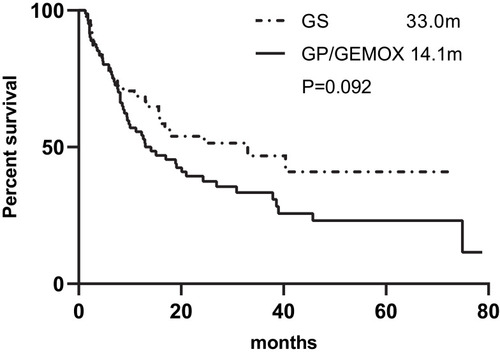
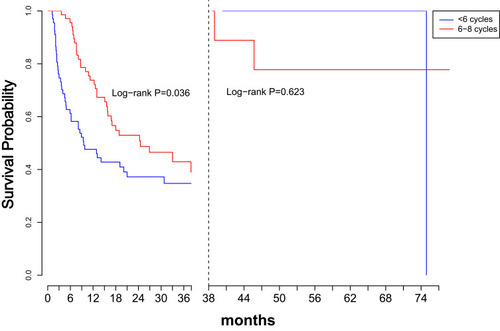
Figure 3 Kaplan–Meier Curves of RFS based on lymph node involvement (A) and chemotherapy cycles (B).
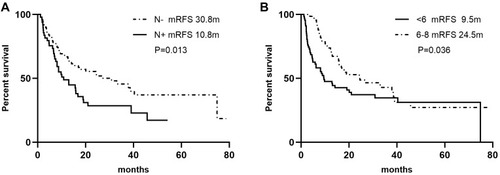
Figure 5 RFS benefits of different chemotherapy regimens in subgroups.
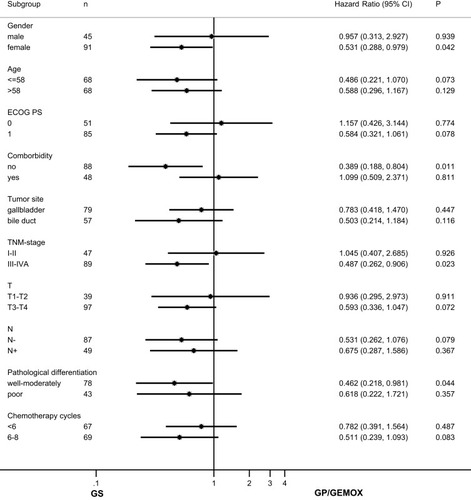
Table 4 Treatment-Related Adverse Events