Figures & data
Table 1 Summary of key demographic characteristics
Table 2 Treatment characteristics and patient status
Figure 1 Kaplan–Meier estimates of survival of 37 patients with nonsquamous NSCLC, stage IIIB/IV, treated with bevacizumab plus chemotherapy. (A) The median event-free survival was 9.4 months (95% CI: 7.1–11.7) and (B) median overall survival was 21.5 months (95% CI: 12.6–30.5).
Abbreviations: CI, confidence interval; NSCLC, non-small-cell lung cancer.
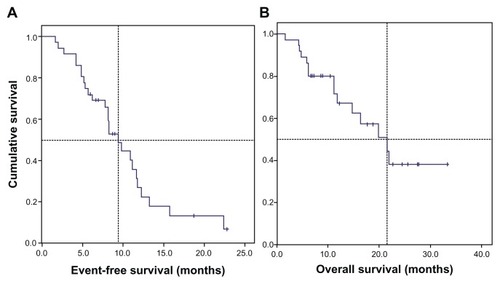
Table 3 Adverse events reported during treatment with chemotherapy plus bevacizumab (grade 3 or higher) according to the Common Terminology Criteria for Adverse Events (version 4.02) of the National Cancer Institute