Figures & data
Figure 1 A498 cells express BK B1 and BK B2 receptors.
Notes: (A) RT-PCR demonstrates the presence of BK B1 and BK B2 mRNA receptors in A498 cells. (B) Western blot analyses of A498 cell lysates (40 μg of total protein) with BK B1 and BK B2 receptor antibodies supporting the expression of BK receptors on a protein level. Antibodies were used at a 1:1000 dilution as per the manufacturer’s recommendations.
Abbreviations: BK, bradykinin; RT-PCR, reverse transcription-polymerase chain reaction.
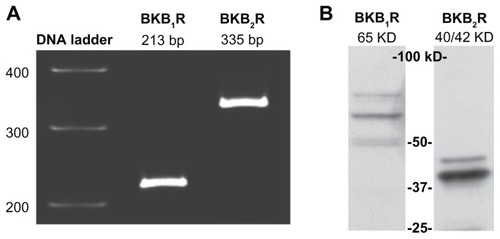
Figure 2 Bradykinin induces elevations in intracellular Ca2+ in A498 cells.
Notes: Cells grown on collagen-coated cover slips were incubated with the calcium-sensitive probe Fluo-4AM, and the detection of calcium-dependent fluorescence was performed with a confocal microscope as described in the Materials and methods section. Changes in fluorescence (intracellular free calcium) were recorded as changes in gray-scale intensity. (A) Shown are representative tracings from two recordings in the presence or absence of the BK B2 receptor antagonist HOE140 (1 μM). (B) Average values of percentage increase over basal in grey-scale intensity subsequent to the addition of BK (100 nM) or the calcium ionophore A23187 (3 μM). Experiments were performed three times.
Abbreviation: BK, bradykinin; RFU, relative fluorescence units.
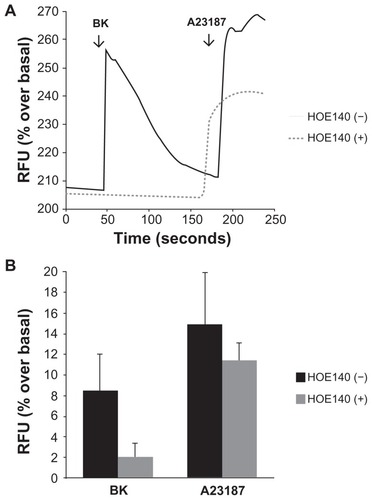
Figure 3 Bradykinin stimulates NHE activity in A498 cells.
Notes: Proton microphysiometry was performed on quiescent A498 cell monolayers as described in the Methods section. Cells were exposed to a perfusate-containing drug (100 nM BK) during the time span encompassed by the gray box. (A) BK (white circles) stimulates ECAR, whereas pretreatment with 5 μM of the NHE-1 and -2 inhibitor 5-(N-methyl-N-isobutyl)-amiloride (MIA) for 30 minutes prior to BK stimulation blocked the effect (black diamonds). (B) BK-induced proton efflux was blocked by preincubation with 1 μM of HOE140, a BK B2 receptor antagonist. The BK B1 receptor antagonist, des-Arg10-HOE140, did not change BK-induced ECAR. Experiments were performed three times. Data are mean + SEM.
Abbreviations: BK, bradykinin; ECAR, extracellular acidification rate; NHE, Na+/H+ exchange; SEM, standard error of the mean.
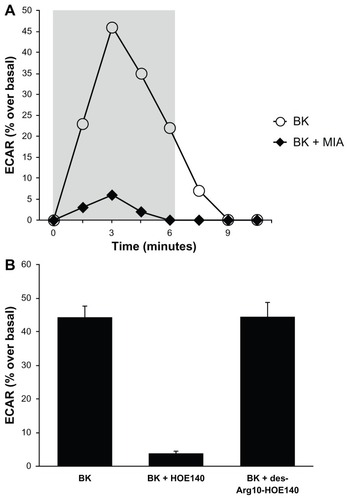
Figure 4 Bradykinin stimulates ERK phosphorylation in A498 cells.
Notes: (A) Time course: A498 cells were stimulated with 100 nM of BK for the indicated periods of time. ERK phosphorylation was measured by immunoblotting with anti-phospho-ERK antibodies, as described in the Materials and methods section. (B) Concentration response: A498 cell were stimulated with the indicated concentrations of BK for 5 minutes. (C) BK-induced ERK phosphorylation is mediated by the B2 receptor: cells were pretreated for 30 minutes with a vehicle, with 1 μM of HOE140, or with 1 μM of des-Arg10-HOE140, then stimulated with 100 nM BK for 5 minutes. Representative phospho-ERK blots are shown. The same blots were stripped and reprobed with antibodies for total ERK to assure the equal loading of a protein sample on a gel (total ERK). Experiments were performed at least three times.
Abbreviations: BK, bradykinin; ERK, extracellular signal-regulated kinase.
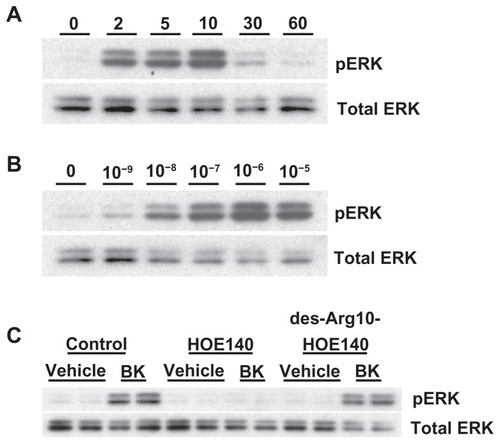
Figure 5 Effects of phospholipase C (PLC), protein kinase C (PKC), Ca2+-, and calmodulin (CaM) inhibitors on BK-induced ERK phosphorylation.
Notes: (A) Cells were preincubated with a vehicle, 10 μM U73122 (a PLC inhibitor), or 10 μM U73343 (negative control) for 30 minutes before stimulation with 100 nM BK for 5 minutes. (B) Cells were pretreated with either 2 μM GF109203X (a PKC inhibitor) for 30 minutes or with 160 nM PMA for 20 hours, followed by either BK 100 nM or PMA 1 μM stimulation for 5 minutes. (C) A498 cells were pretreated for 30 minutes with a vehicle, 10 μM of BAPTA (an intracellular Ca2+ chelator), or 50 μM of W7 (a CaM inhibitor), and then stimulated for 5 minutes with 100 nM BK. (D) BK-induced ERK phosphorylation does not depend on EGFR tyrosine kinase activity: cells were pretreated for 30 minutes with a vehicle, with 1 μM of AG1478 (an EGFR kinase inhibitor) before stimulation with 100 nM BK, or with 10 ng/mL EGF for 5 minutes. ERK phosphorylation was detected by immunoblotting with an anti-phospho-ERK antibody. Bars represent intensities of phospho-ERK bands relative to total ERK expressed as a fold of basal of control (cells treated with a vehicle). Insets show representative phospho-ERK blots. The same blots were stripped and reprobed with antibodies for total ERK to assure the equal loading of a protein sample on a gel (total ERK). Experiments were performed three times in duplicate. Data are presented as mean + SEM. Statistical probability in figures is expressed as *P < 0.05, and as **P < 0.01 versus vehicle-treated samples.
Abbreviations: BK, bradykinin; ERK, extracellular signal-regulated kinase; PMA, phorbol 12-myristate 13-acetate; SEM, standard error of the mean.
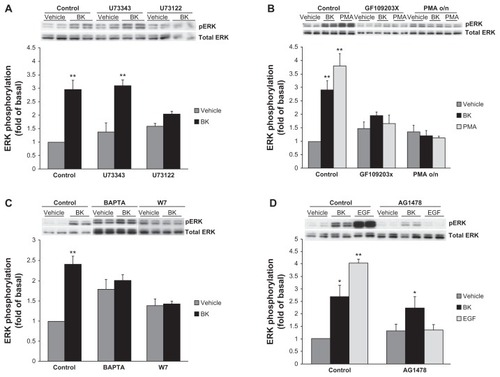
Figure 6 NHE activity is required for bradykinin-induced ERK activation.
Notes: (A) BK-induced NHE activity does not require ERK activation. Cells were pretreated with either 10 μM of an MEK inhibitor (PD98059) or with 5 μM of an NHE inhibitor (MIA) for 30 minutes prior to the application of 100 nM of BK for four measurement cycles, and ECAR was assessed by proton microphysiometry as described in the Materials and methods section. Experiments were performed at least three times. The data are means + SEM. (B) BK-induced ERK phosphorylation is NHE-dependent. Cells were pretreated with 10 μM PD98059 or with 5 μM MIA for 30 minutes prior to stimulation with 100 nM BK for 5 minutes. Bars represent the intensities of phospho-ERK bands relative to the total ERK expressed as a fold of control (cells treated with a vehicle). Experiments were performed three times in duplicate. Data are presented as means + SEM. (C) Bradykinin stimulates the proliferation of A498 cells. A498 cells were preincubated for 1 hour with a vehicle, or with 10 μM PD98059 or 5 μM MIA before the addition of 100 nM BK or 20% fetal bovine serum (positive control) or a vehicle (negative control) for 24 hours. After incubation with a BrdU label for an additional 24 hours at 37°C, the BrdU cell proliferation assay was performed according to the manufacturer’s protocol. Experiments were performed at least three times in triplicate. Data are presented as means + SEM. Statistical probability in figures is expressed as *P < 0.05, and as **P < 0.01 versus vehicle-treated samples.
Abbreviations: BK, bradykinin; ECAR, extracellular acidification rate; ERK, extracellular signal-regulated kinase; MEK, mitogen- and extracellular signal-regulated kinase; MIA, 5-(N-methyl-N-isobutyl)-amiloride; NHE, Na+/H+ exchange; SEM, standard error of the mean.
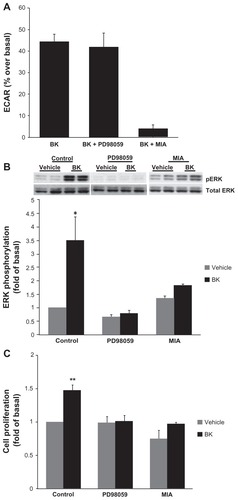
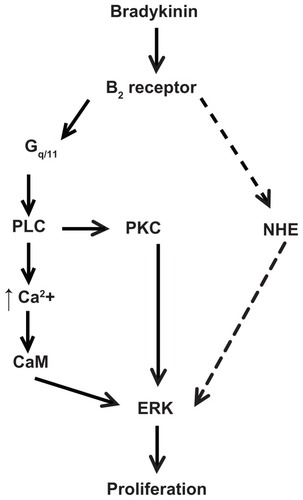
Figure 7 Scheme of proposed signaling pathway used by bradykinin to stimulate proliferation in A498 cells. This scheme is described in the Discussion section.
Notes: BK stimulates ERK through the B2 receptor-Gq/11 pathway, which includes the activation of phospholipase C (PLC) and protein kinase C (PKC), PLC-dependent Ca2+ mobilization, and calmodulin (CaM) activity. BK B2 receptors stimulate NHE1 activity, which is necessary for the ERK-dependent proliferation of A498 cells. The dashed line indicates a putative pathway used by BK to activate NHE upstream of ERK.
Abbreviations: BK, bradykinin; ERK, extracellular acidification rate; NHE, Na+/H+ exchange.