Figures & data
Figure 1 Neovascularization related proteins are highly expressed in LGACC tissues. VEGF (A), VEGFR1 (C), VEGFR2 (E), and FGFR1 (G) expression in LGACC and normal tissues detected by immunohistochemistry, and the related statistical analysis (B, D, F and H). ***p < 0.001. n=5.
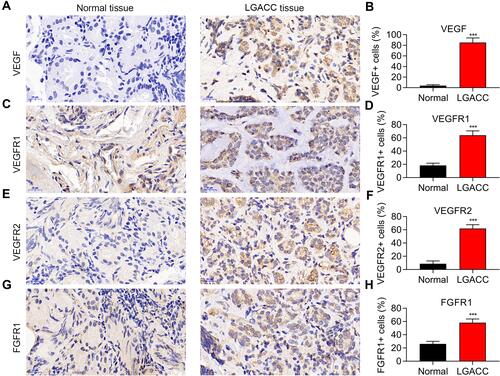
Table 1 Characteristics of Patients with LGACC
Figure 2 Patient-derived xenograft (PDX) model characterization. (A) Hematoxylin and Eosin staining of PDX tissue. (B–D) VEGF (B), VEGFR1 (C), and VEGFR2 (D) expression in the PDX model detected by immunohistochemical staining. (E) The statistical analysis of (B–D). n=5.
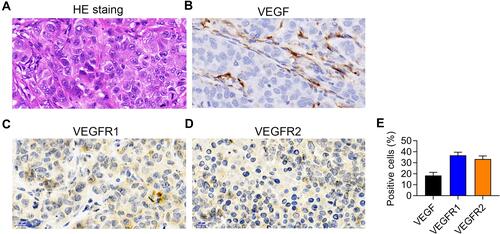
Figure 3 The efficacy of bevacizumab and cisplatin on patient-derived xenograft (PDX) tumor growth and body weight of mice. (A) Representative images of PDX tumor in vehicle, bevacizumab treatment, and cisplatin treatment groups. (B) PDX tumor weight in vehicle, bevacizumab treatment, and cisplatin treatment groups. **p < 0.01; NS, not significant. (C) PDX tumor growth curves. (D) Body weight of PDX mice following vehicle, bevacizumab, and cisplatin treatment. **p < 0.01; ***p < 0.001. n=7.
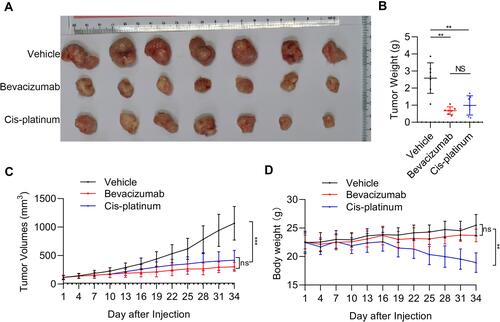
Figure 4 Bevacizumab and cisplatin suppress angiogenesis and cell proliferation in patient-derived xenograft (PDX) tumors. (A–E) Expression of Ki-67 (A), CD34 (B), P53 (C), VEGF (D), and VEGFR2 (E) detected by immunohistochemical staining in vehicle, bevacizumab treatment, and cisplatin treatment groups. *p < 0.01; **p < 0.05; ***p < 0.001. n=5.
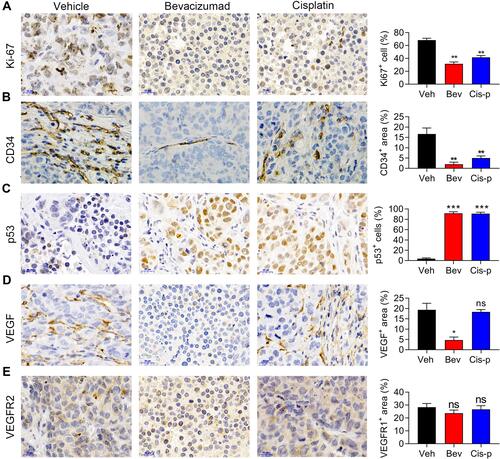
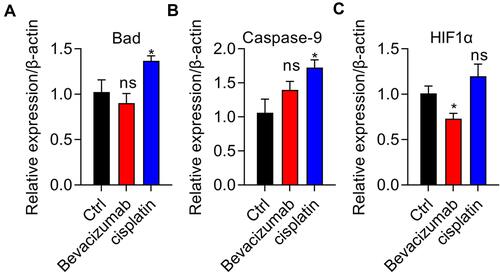
Figure 5 Cisplatin elevates apoptotic gene expression and bevacizumab suppresses HIF1α expression. (A and B) Proapoptotic-related mRNA expression of genes Bad (A) and Caspase-9 (B) was assessed using quantitative real-time PCR in vehicle, bevacizumab, and cisplatin treatment groups. *p < 0.01. (C) Examination of HIF1α expression using quantitative real-time PCR in vehicle, bevacizumab treatment, and cisplatin treatment groups. *p < 0.01. n=3.