Figures & data
Table 1 Efficacy of Asciminib by Months 6 and 12
Figure 1 ASCEMBL study design.
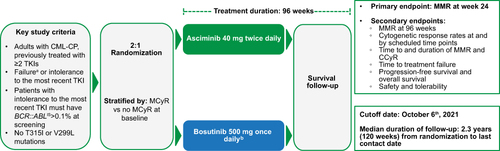
Figure 2 Risk difference (95% CI) for MMR at week 96 from subgroup analyses.
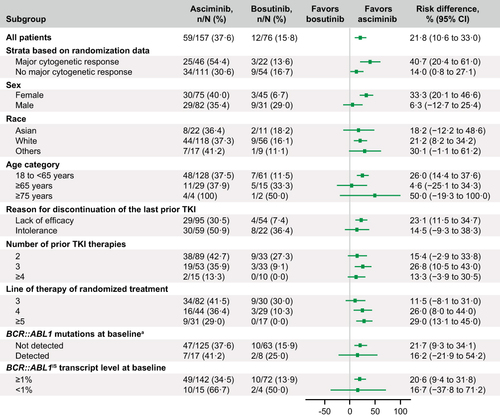
Table 2 Most Frequent Adverse Events (≥10% of Patients in Any Treatment Arm) by Week 24
Table 3 Ongoing Trials of Asciminib in CML
