Figures & data
Figure 1 Patient selection diagram. Pooled data from 240 patients with non-small cell lung cancer (NSCLC) who received carboplatin (CBDCA)-based chemotherapy regimens (CBDCA + pemetrexed [PEM]/CBDCA + paclitaxel [PTX]) were selected among 2468 patients from two prospective observational studies.
![Figure 1 Patient selection diagram. Pooled data from 240 patients with non-small cell lung cancer (NSCLC) who received carboplatin (CBDCA)-based chemotherapy regimens (CBDCA + pemetrexed [PEM]/CBDCA + paclitaxel [PTX]) were selected among 2468 patients from two prospective observational studies.](/cms/asset/98c543a2-36e8-48a1-80d2-3a7d43facc4b/dcmr_a_12197443_f0001_c.jpg)
Table 1 Patients’ Baseline Characteristics
Table 2 Unweighted and Weighted Baseline Characteristics of Patients with NSCLC Treated with CBDCA Categorized by the Number of Antiemetic Regimens
Figure 2 Incidence of delayed nausea and vomiting. The incidence of delayed nausea and vomiting was significantly higher in the two-antiemetic group than that in the three-antiemetic group.
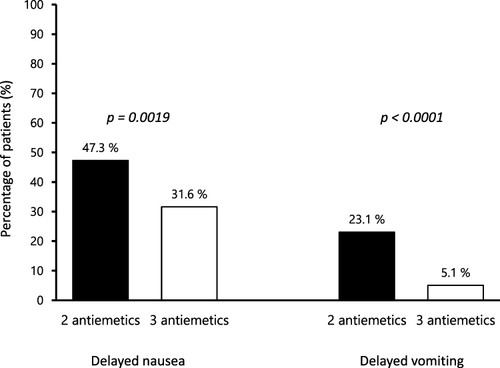
Table 3 Risk Factors for Delayed Nausea and Delayed Vomiting
