Figures & data
Figure 1 Leukocytes (WBC) and lymphocytes (Ly) trend during the treatment with mogamulizumab. Basal value: 12/02/2020. First cycle of mogamulizumab: 12/23/2020. Second cycle of mogamulizumab on 01/21/2021. End of mogamulizumab: 06/01/2021.
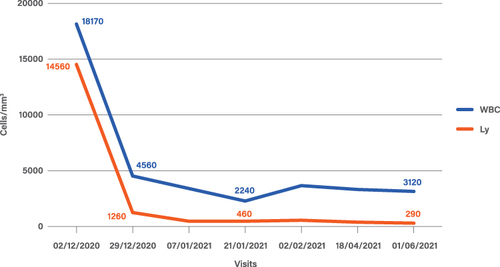
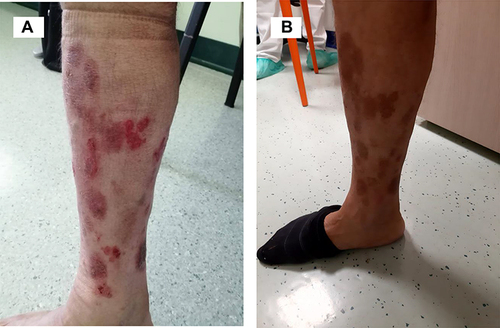
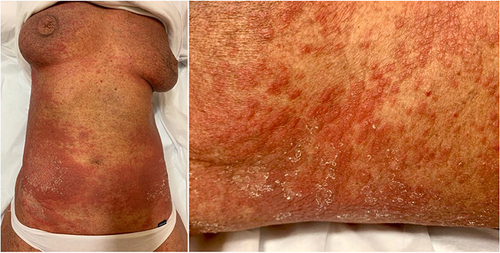
Figure 3 Diffuse erythema with scaling covering approximately 60% of BSA, onychodystrophy, ectropion, mild palmo-plantar hyperkeratosis.
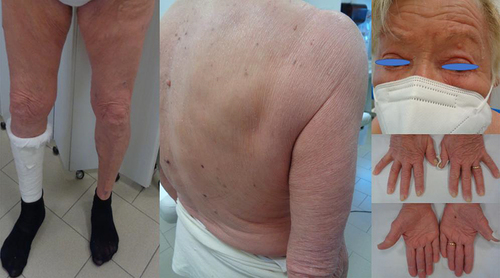
Figure 4 Epidermotropic infiltrate with Pautrier’s microabscesses with atypical lymphocytes and a patchy lichenoid infiltrate in the papillary dermis.
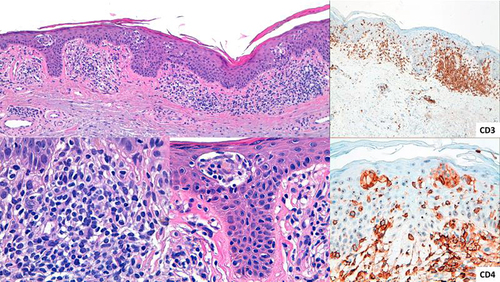
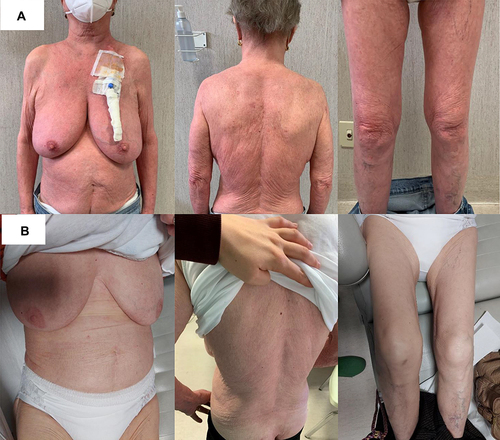
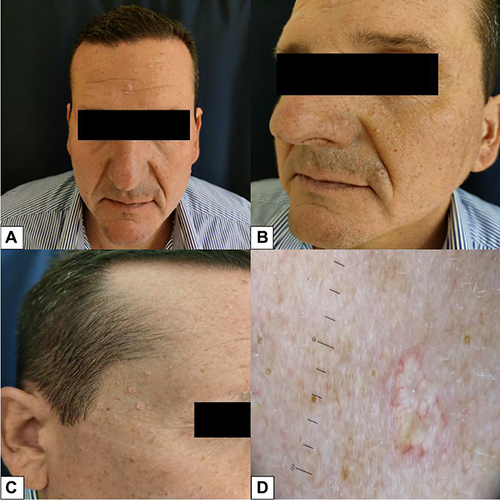
Figure 5 Already after the first cycle of mogamulizumab the patient achieved prompt clinical response with >90% clearance of skin disease.
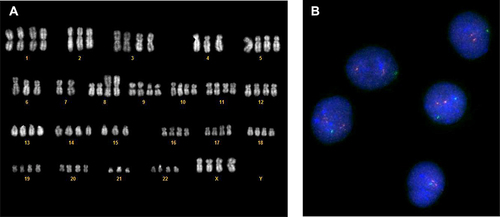
Figure 7 Cytogenetic abnormalities before and after mogamulizumab administration. After the first course of mogamulizumab, the hypothetraploid clone showed by karyotype and confirmed by FISH (A) was cleared and only a second clone remained detectable by FISH (B).