Figures & data
Table 1 Baseline patient characteristics and previous therapy before administration of sorafenib
Figure 1 Overall survival for all patients.
Note: Median overall survival was 11.8 (range 7–763) days.
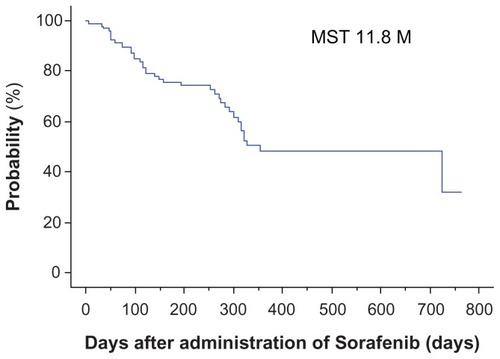
Figure 2 Relationship between continuation of administration and overall survival.
Note: In the group that continued administration for ≥90 days, overall survival was significantly higher than in the group that discontinued administration within 90 days.
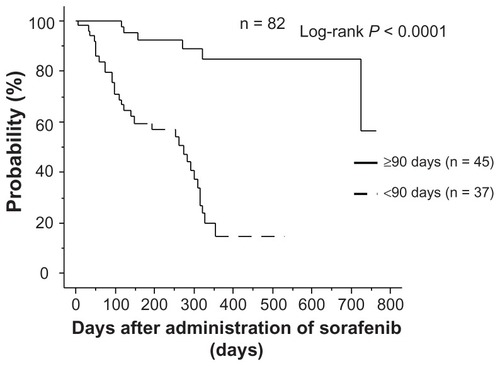
Figure 3 Relationship between continuation of administration and overall survival in patients receiving 200 mg or 400 mg.
Note: In the group that continued administration for ≥90 days, overall survival was significantly higher than in the group who discontinued administration within 90 days. and 3). Therefore, we concluded that treatment with sorafenib for ≥90 days achieves better overall survival, even at a reduced dose.
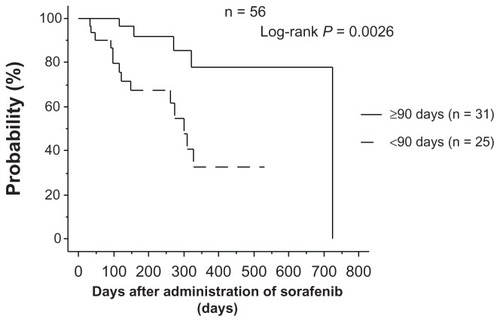
Table 2 Reasons for discontinuation of sorafenib within 90 days
Table 3 Risk factors contributing to overall survival (n = 96)
Table 4 Risk factors contributing to discontinuation of sorafenib administration within 90 days (n = 82)