Figures & data
Figure 1 The patient screening process: screening EC patients received PD-1 inhibitors in our center, 800 patients received PD-1 inhibitors were screed, excluded 576 patients without OMEC. 224 patients with OMEC received PD-1 inhibitors were screed, 11 patients with other active synchronous carcinomas were excluded. Subsequently, 213 patients with OMEC received PD-1 inhibitors were screed, 127 patients who only received systemic therapy were excluded. Eventually, 86 patients with OMEC who received radiotherapy plus PD-1 inhibitors were included in this study.
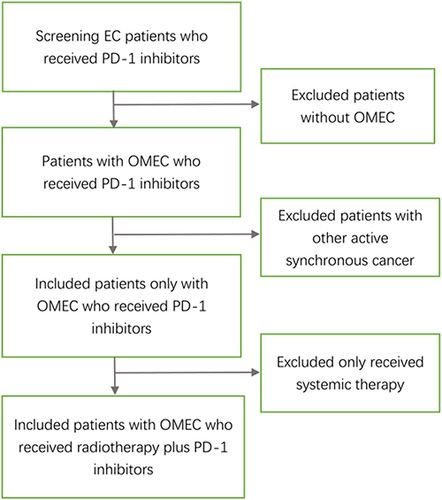
Table 1 Baseline Characteristics (n=86)
Table 2 Details of Distant Metastatic Sites (n=228)
Table 3 Response to Treatment
Figure 2 (A) The median PFS of all patients was 15.2m (95% CI, 12.1–18.2), 1-year and 2-year PFS rate were 61.4% and 26.7%; (B) The median PFS of SO (15.1m, 11.7–18.5) and MO (16.3m, 12.0–20.6) patients (p=0.63).
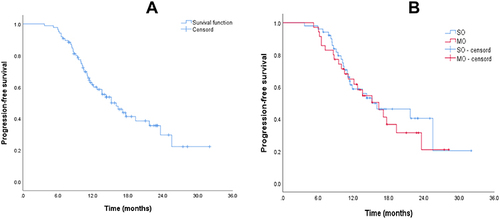
Table 4 Failure Patterns in Full-Analysis Population
Table 5 Treatment-Related Adverse Events
