Figures & data
Figure 1 Osteoclasts in the osteosarcoma microenvironment. Osteosarcoma cells and osteoblasts in the tumor microenvironment affect osteoclasts through direct and indirect ways, leading to changes in osteoclasts. Osteoclasts produce related factors, which in turn regulate osteosarcoma cells and osteoblasts, and then activated or stimulated osteosarcoma cells and osteoblasts affect osteoclasts in a direct or indirect way, forming a vicious cycle. Osteosarcoma cells and tumor-associated fibroblasts melt in the osteosarcoma microenvironment and undergo multi-nucleated osteoclast-like changes. The myeloid-derived suppressor cells produce high levels of HIF-1 in hypoxia environment, thus producing NO-mediated osteoclast differentiation, and NO can also mediate HIF-1 increase through PI3K and AKT pathways, thus forming a positive feedback.
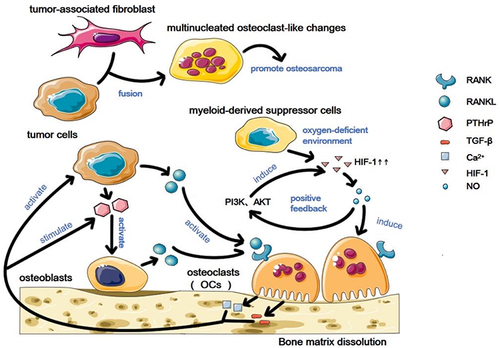
Figure 2 Osteoclasts in the bone marrow microenvironment. In the bone marrow microenvironment, stimulated by RANKL and M-CSF, TLR2 binds to SIGLEC-15, resulting in the fusion of mononuclear macrophages into polykaryotes and the final differentiation into osteoclasts. T cell expression of TNFSF11 encoding RANKL can also activate osteoclasts. In addition, DCs has been found in advanced bone metastases from breast cancer to differentiate into DC-OCs upon contact with T cells. Such osteoclasts can secrete IL-23, and binding to IL-23R on T cells also promotes the production of activated osteoclasts in RANKL.
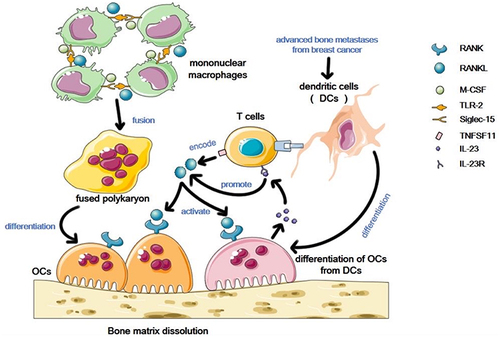
Table 1 Interactions Between Osteoclasts and Other Cells in the Osteosarcoma Microenvironment
Table 2 Osteosarcoma Treatment Related Clinical Trials
