Figures & data
Figure 1 Schematic of HER2 signaling system.
Notes: HER2 homo- or heterodimers predominantly activate to major pathways, namely the RAS/MAP kinase and PI3K/AKT pathways, which drive the aberrant proliferation and survival of breast cancer cells. Inset depicts the major tyrosine residues phosphorylated upon HER2 activation, as well as the predominant downstream signaling system coupled to these events. See “Cell signaling mediated by HER” for additional details.
Abbreviations: Bcl-2, B-cell CLL/lymphoma 2; ERK, extracellular signal-regulated kinase; Grb2, growth factor receptor-bound protein 2; HER, human epidermal growth factor receptor; MEK, mitogen-activated protein kinase kinase; mTORC, mammalian target of rapamycin complex; P, phosphorylation site; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5-trisphosphate; PTEN, phosphatase and tensin homologue; SOS, son of sevenless.
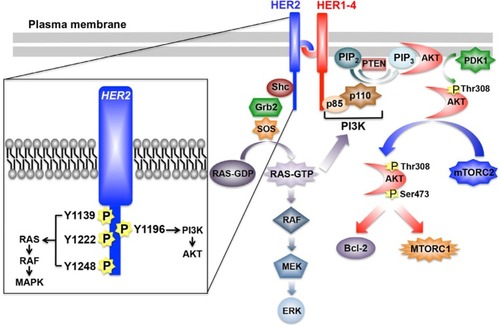
Figure 2 Schematic of trastuzumab- and pertuzumab-mediated inhibition of HER2 receptor activation.
Notes: The extracellular domains of HER family members comprise of four domains (I–IV) that regulate ligand binding (domains I and III) and receptor dimerization (domain II). Trastuzumab (green antibody) targets domain IV and alleviates ligand-independent signaling (A), whereas pertuzumab (blue antibody) targets domain II and alleviates dimer and ligand-dependent signaling (B). Combining pertuzumab with trastuzumab produces the best pCR rates by inhibiting all receptor activation states (C).
Abbreviations: HER, human epidermal growth factor receptor; pCR, pathological complete response; pert, pertuzumab; trast, trastuzumab.
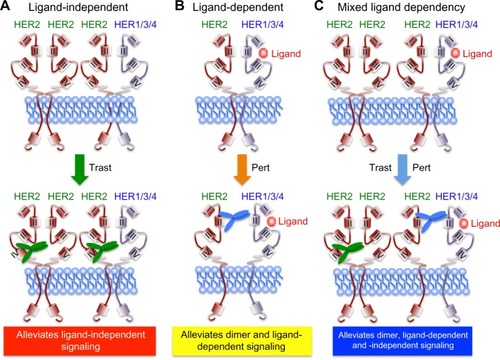
Figure 3 Results from the NeoSphere trial comparing pCR rates by experimental treatment arm.
Note: Adapted from Lancet Oncol. Vol 13(1). Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Pages 25–32. Copyright 2012, with permission from Elsevier.Citation41
Abbreviations: DP, docetaxel and pertuzumab; DT, docetaxel and trastuzumab; DTP, docetaxel, trastuzumab, and pertuzumab; pCR, pathological complete response; TP, trastuzumab and pertuzumab.
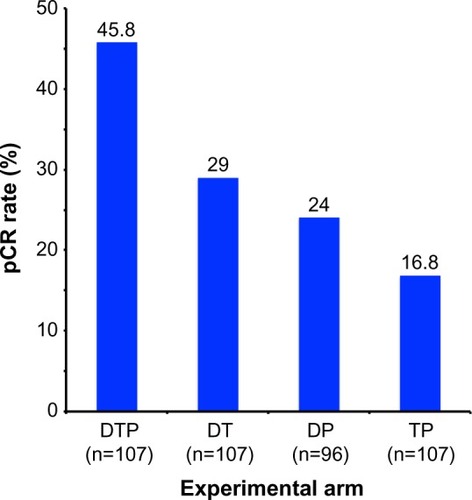
Figure 4 Results from the NeoSphere trial comparing pCR rates by experimental treatment arm and hormone receptor status.
Note: Adapted from Lancet Oncol. Vol 13(1). Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadpertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Pages 25–32. Copyright 2012, with permission from Elsevier.Citation41
Abbreviations: DP, docetaxel and pertuzumab; DT, docetaxel and trastuzumab; DTP, docetaxel, trastuzumab, and pertuzumab; pCR, pathological complete response; TP, trastuzumab and pertuzumab; ER, estrogen receptor; PR, progesterone receptor.
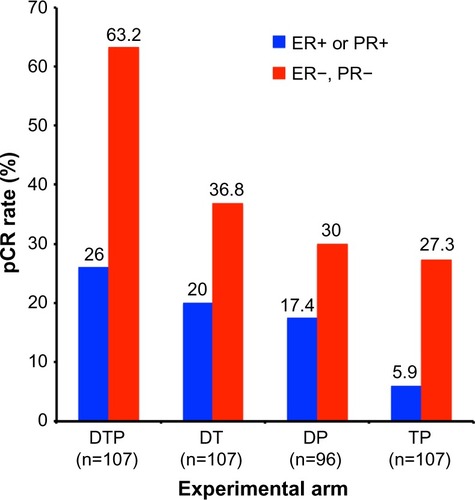
Figure 5 Results from the TRYPHAENA trial comparing pCR rates by experimental treatment arm.
Note: Adapted from Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–2284, by permission of Oxford University Press.Citation44
Abbreviations: DTP, docetaxel, trastuzumab, and pertuzumab; DTP w/Carb, DTP with carboplatin; FEC w/DPT, fluorouracil, epirubicin, and cyclophosphamide with concurrent DTP; FEC -> DTP, FEC followed by DTP; pCR, pathological complete response.
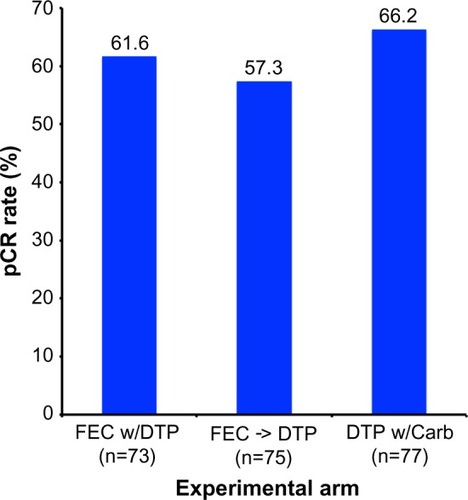
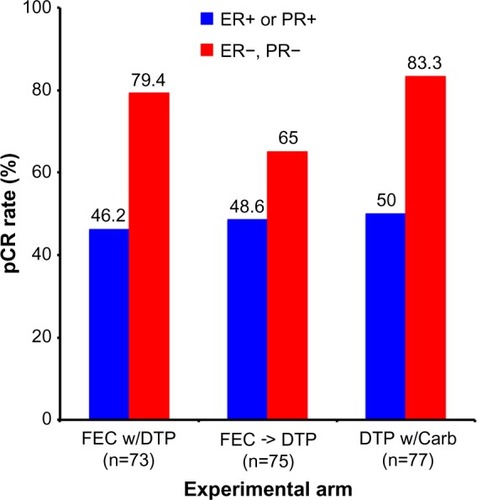
Figure 6 Results from the TRYPHAENA trial comparing pCR rates by experimental treatment arm and hormone receptor status.
Note: Adapted from Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–2284, by permission of Oxford University Press.Citation44
Abbreviations: DTP, docetaxel, trastuzumab, and pertuzumab; DTP w/Carb, DTP with carboplatin; FEC w/DPT, fluorouracil, epirubicin, and cyclophosphamide with concurrent DTP; FEC -> DTP, FEC followed by DTP; pCR, pathological complete response; ER, estrogen receptor; PR, progesterone receptor.