Figures & data
Figure 1 Model of osteoclastogenesis during bone homeostasis and tumor cell metastasis to bone. A) In normal bone, RANKL and m-CSF are produced primarily by osteoblasts. m-CSF binds to its receptor c-FMS, expressed on osteoclast progenitors, and RANKL binds to its receptor on pre-osteoclasts to promote osteoclastogenesis. Osteoprotegrin, also produced by osteoblasts, acts as a decoy receptor for RANKL and negatively regulates osteoclast differentiation. In this model, osteoblast and osteoclast activity are in homeostasis through careful regulation of osteoclastogenesis. B) When cancer cells metastasize to the bone, increased IL-6 may be produced by both the cancer cells and the osteoblasts, as an inflammatory response to the cancer cells. IL-6 then stimulates various types of stromal cells in the bone, which include bone marrow cells, osteoblasts, and fibroblasts in the area of the metastasis, to increase the expression of RANKL and m-CSF by osteoblasts. This IL-6-mediated increase in RANKL and m-CSF also occurs with injury and inflammation to the bone, but unlike in cancer metastasis, it is transient. RANKL and m-CSF then, in turn, activate the osteoclast differentiation cascade, where m-CSF strongly stimulates early stages of osteoclast differentiation, and RANKL stimulates late stages of osteoclast differentiation, as well as osteoclast activity. Once this occurs, osteoclast activity becomes dysregulated and reduces bone integrity.
Abbreviations: c-FMS, colony stimulating factor 1 receptor; IL-6, interleukin 6; IL-6R, IL-6 receptor; m-CSF, macrophage-colony stimulating factor; RANKL, receptor activator of nuclear factor κB ligand; sRANKL, soluble form of RANKL.
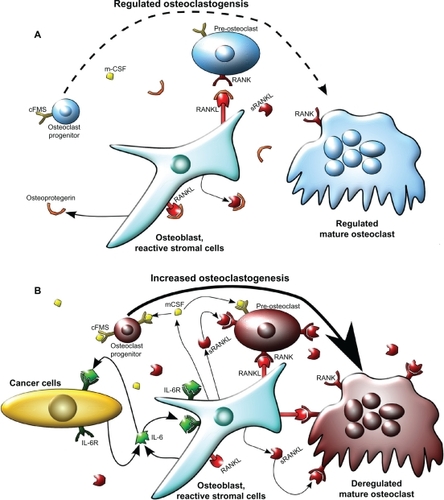
Figure 2 Factors that increase IL-6 production in response to various stimuli. Increased IL-6 production is associated with stimuli such as infection and inflammation. Infection, injury, and cancer can all stimulate inflammation that can lead to the increase of IL-6-modulating factors such as IL-1β, COX-2, PGE2, and TGF-β. Infection can also promote LPS secretion from bacteria, which increases NF-κB-dependent IL-6 levels. There are two main IL-6 production pathways: NF-κB-dependent and NF-κBindependent. NF-κB-independent pathways upregulate IL-6 secretion via TGF-β or PGE2, which is produced downstream of COX-2 activation. In the NF-κB-dependent pathway, LPS or IL-1β stimulate NF-κB activity that causes an increase in IL-6 production.
Abbreviations: COX-2, cyclooxygenase 2; IL-1β, interleukin 1β; IL-6, interleukin 6; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; PGE2, prostaglandin E2; TGF-β, transforming growth factor β.
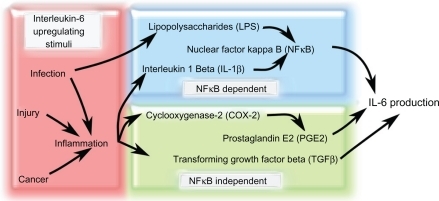
Figure 3 Model of canonical IL-6 signaling versus IL-6 trans-signaling in tumor progression and metastases. In the canonical IL-6 signaling pathway, the IL-6 receptor subunit is membrane bound and forms a heterotrimer with two gp130 subunits. When IL-6 binds to the receptor, STAT3 is activated in a JAK-dependent manner that leads to increased RANKL expression. IL-6 may also activate AKT via increased JAK-dependent PI3K activity and result in cell survival and anti-apoptosis signaling. Concomitantly, increased MAPK activity downstream of JAK activation can lead to upregulated cell growth, proliferation, and mitosis. In the IL-6 trans-signaling pathway, IL-6 first binds to the truncated sIL6R. The IL-6/sIL6R complex then binds to the membrane-bound gp130 dimer to form an IL-6 trans-signaling complex. Due to the fact that the sIL-6R lacks a membrane signaling domain, there appears to be significant differences in the intracellular signaling pathways. While IL-6 trans-signaling also leads to phosphorylation and activation of STAT3, increased cell survival, proliferation, and mitosis occurs in an AKT-and MAPK-independent manner. The exact mechanisms for IL-6 trans-signaling leading to increased cell survival, proliferation, and mitosis are not yet known.
Abbreviations: IL-6, interleukin 6; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; RANKL, receptor activator of nuclear factor κB ligand; sIL6R, soluble IL-6 receptor; STAT3, signal transducer and activator of transcription 3.
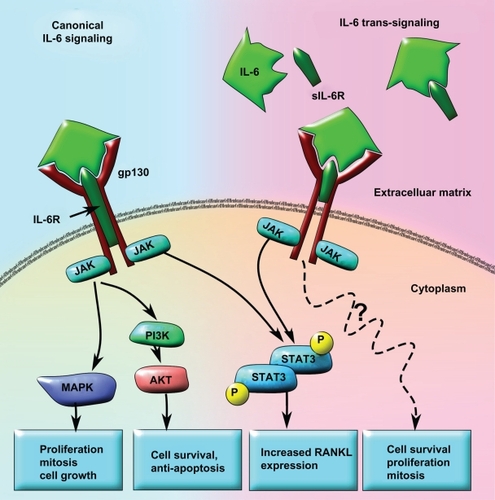
Table 1 Targeted therapies for IL-6