Figures & data
Table 1 Study selection criteria for inclusion in the network meta-analysis
Figure 1 PRISMA diagram showing selection of LABA monotherapy trials.
Notes: aSearch was performed for abstracts published between January 1, 2013 and March 24, 2015. bPrior search was performed for abstracts published between 1989 and January 1, 2013.
Abbreviations: LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; RCT, randomized controlled trial.
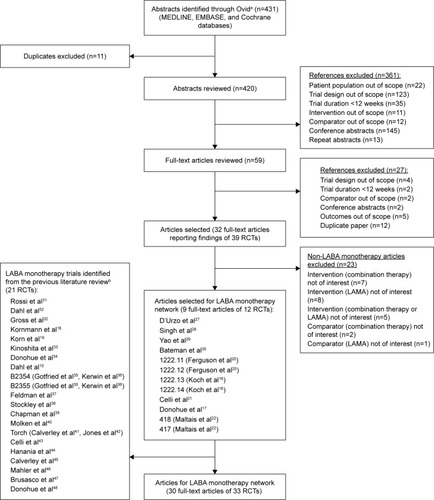
Figure 2 Network diagram of LABA monotherapy trials included in network meta-analysis.
Abbreviations: BID, twice daily; OD, once daily; LABA, long-acting beta agonist.
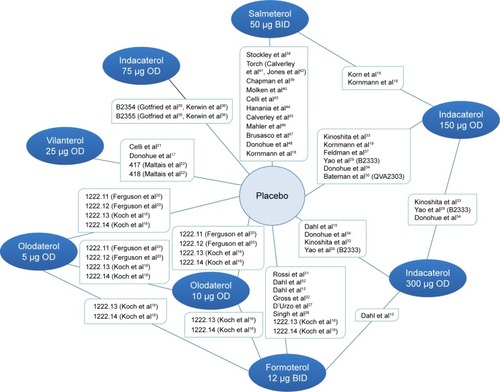
Figure 3 Change from baseline differences in trough FEV1 (L) for intervention versus placebo at 12 and 24 weeks. (A) Trough FEV1 at 12 weeks and (B) trough FEV1 at 24 weeks.
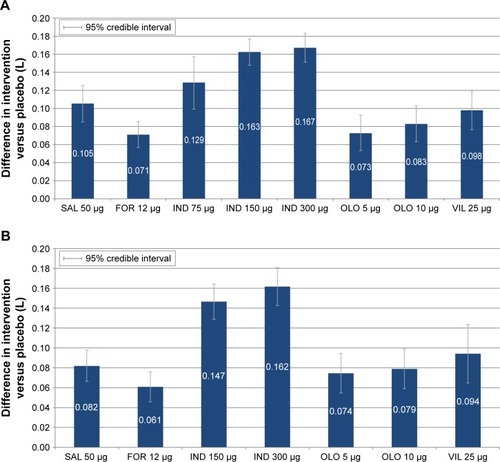
Table 2 Results of random effect network meta-analysis for change from baseline in trough FEV1 (L) at 12 and 24 weeks
Table 3 Results of the random effect network meta-analysis for change from baseline in TDI focal score at 12 and 24 weeks
Figure 4 Difference in change from baseline TDI focal score of intervention versus placebo at 12 and 24 weeks. (A) TDI focal score at 12 weeks and (B) TDI focal score at 24 weeks.
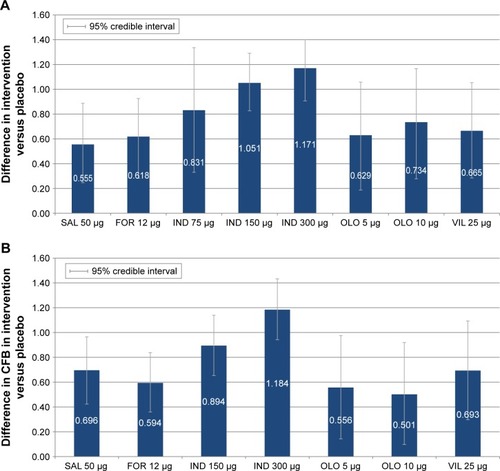
Table 4 Results of the random effect network meta-analysis for change from baseline in SGRQ total score at 12 and 24 weeks
Figure 5 Change from baseline difference in SGRQ total score for intervention versus placebo at 12 and 24 weeks. (A) SGRQ total score at 12 weeks and (B) SGRQ total score at 24 weeks.
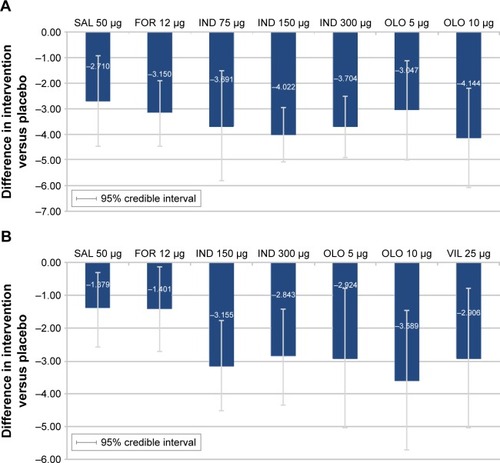
Table 5 Results of random effect network meta-analysis for exacerbation rate
Figure 6 Exacerbation rate (rate ratio) of intervention versus placebo.
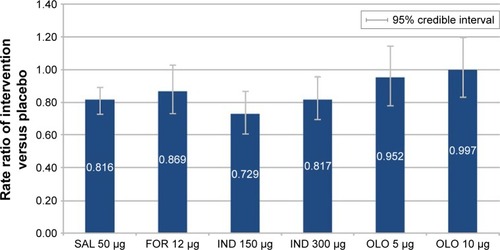
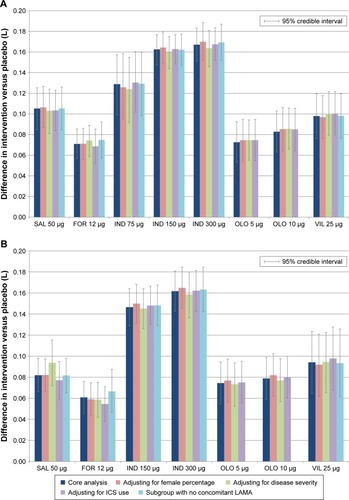
Figure 7 Sensitivity analysis for change from baseline in trough FEV1 (L). (A) Trough FEV1 at 12 weeks and (B) trough FEV1 at 24 weeks.