Figures & data
Figure 1 Structure of the decision model used.
Abbreviation: FEV1, forced expiratory volume in 1 second.
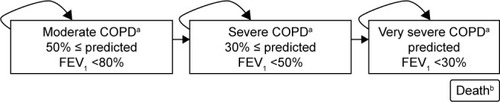
Table 1 Clinical efficacy, including change in trough FEV1, exacerbations, and AEs
Table 2 Costs included as model inputs and the utility values for COPD severity and exacerbation severity
Table 3 Base-case analysis results over a lifetime horizon
Figure 2 Cost-effectiveness of UMEC/VI treatment in patients with moderate to very severe COPD: probabilistic sensitivity analyses (A) UMEC/VI compared with TIO and (B) UMEC/VI compared with no long-acting bronchodilator.
Abbreviations: CE, cost-effectiveness; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; TIO, tiotropium; UMEC, umeclidinium; VI, vilanterol.
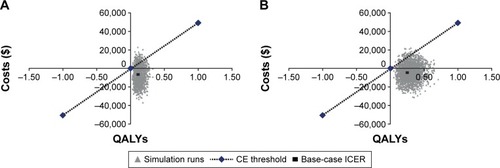
Table S1 Transition probabilities for UMEC/VI
Table S2 Transition probabilities for tiotropium bromide
Table S3 Transition probabilities for open dual LAMA + LABA
Table S4 Transition probabilities for no long-acting bronchodilator
