Figures & data
Table 1 Baseline characteristics of the patients who continued or discontinued roflumilast use
Figure 1 Process of patient recruitment.
Abbreviation: BMI, body mass index.
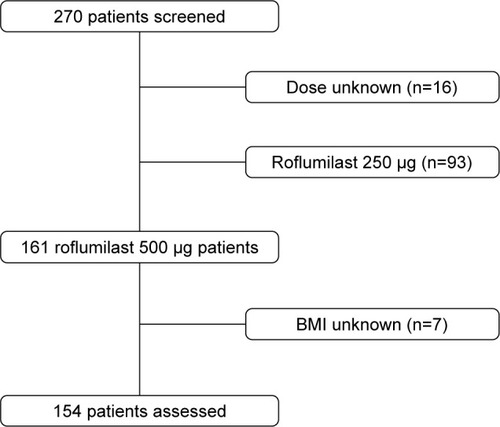
Figure 2 Rates of roflumilast discontinuation by BMI group.
Abbreviation: BMI, body mass index.
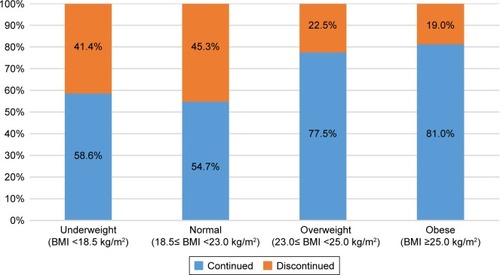
Table 2 Factors associated with roflumilast discontinuation (univariate analysis)
Table 3 Factors associated with roflumilast discontinuation (multi variate analysis)
Table 4 Types of adverse events in patients who continued or discontinued roflumilast use
Table 5 Comparison of patient characteristics by number of adverse events
Table 6 Factors associated with roflumilast adverse events (univariate analysis)
Figure 3 Rates of roflumilast adverse events by BMI group.
Abbreviation: BMI, body mass index.
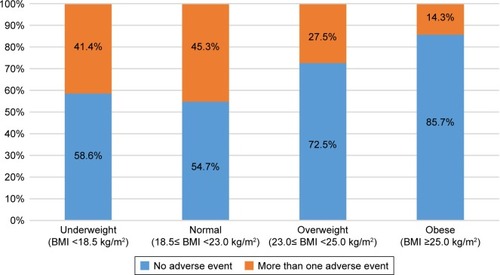
Table 7 Factors associated with roflumilast adverse events (multivariate analysis)
