Figures & data
Table 1 Commonly Used Long-Acting Anticholinergics For Maintenance Treatment Of COPDCitation1
Figure 1 Structural formulas of once-daily LAMAs.Citation19–Citation21
Table 2 Summary Of Trials Evaluating Efficacy And Safety Of Revefenacin
Figure 2 Peak FEV1 treatment difference from placebo in single-dose (A) and multi-dose 7-day (B) trials. Peak FEV1 is the highest value obtained between 0 and 6 hrs after the first dose. *p<0.001. Data are least squares mean±95% confidence interval treatment difference from placebo. Dotted line indicates minimal clinically important difference.Citation31
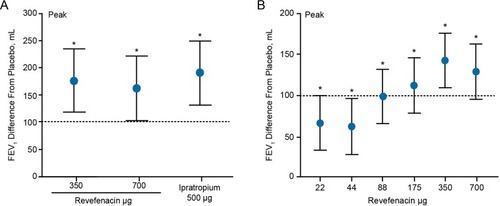
Figure 3 Sustained increase in trough FEV1 for 85 days in two randomized, double-blind, placebo-controlled Phase III trials in patients with moderate to severe COPD (pooled data from NCT2459080 and NCT2512510; N=1,255). Dotted line indicates minimal clinically important difference.Citation31
Abbreviations: LS, least squares; REV, revefenacin; SE, standard error.
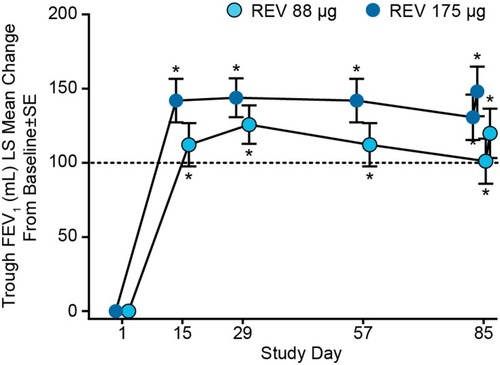
Figure 4 Placebo-adjusted changes from baseline at day 85 trough FEV1 in patients with COPD who received once-daily revefenacin (88 and 175 μg) for nebulization.Citation28 *p<0.001 vs placebo. Day 85 trough FEV1 was the average of values obtained at 23.25 and 23.75 hrs following the 84th dose. Dotted line indicates minimal clinically important difference.Citation31
Abbreviations: LS, least squares; OTE, overall treatment effect; REV, revefenacin; SE, standard error.
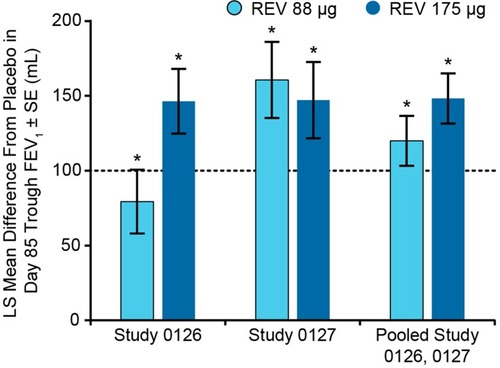
Table 3 Incidence (n, %) Of Treatment-Emergent AEs Reported In ≥2% Patientsa Receiving Revefenacin 175 μg Once Daily In Phase II And III Clinical Trials
