Figures & data
Table 1 Patient demographics and baseline characteristics (pooled safety population)
Figure 1 Efficacy endpoints at week 24 analyzed by airflow obstruction severity (moderate vs severe).
Abbreviations: AB, aclidinium bromide; EXACT, EXAcerbations of Chronic pulmonary disease Tool; FF, formoterol fumarate; HCRU, healthcare resource utilization; ITT, intent-to-treat; LS, least squares; PBO, placebo; TDI, Transition Dyspnea Index.
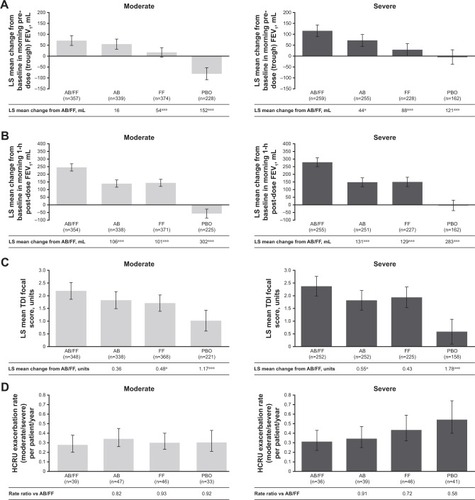
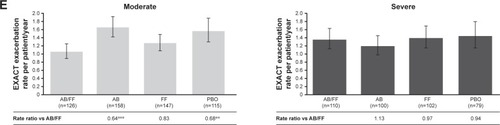
Figure 2 Efficacy endpoints at week 24 analyzed by patient age (<65 vs ≥65 years).
Abbreviations: AB, aclidinium bromide; EXACT, EXAcerbations of Chronic pulmonary disease Tool; FF, formoterol fumarate; HCRU, healthcare resource utilization; ITT, intent-to-treat; LS, least squares; PBO, placebo; TDI, Transition Dyspnea Index.
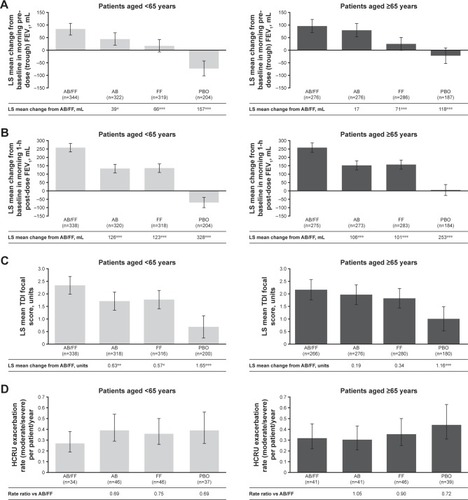
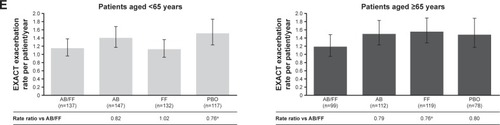
Figure 3 Efficacy endpoints at week 24 analyzed by patient sex.
Abbreviations: AB, aclidinium bromide; EXACT, EXAcerbations of Chronic pulmonary disease Tool; FF, formoterol fumarate; HCRU, healthcare resource utilization; ITT, intent-to-treat; LS, least squares; PBO, placebo; TDI, Transition Dyspnea Index.
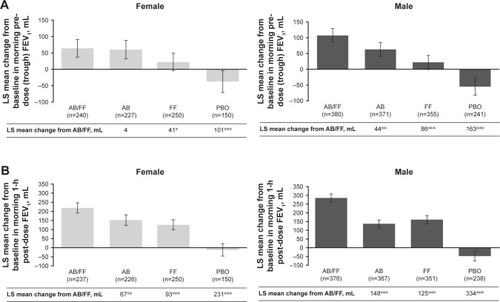
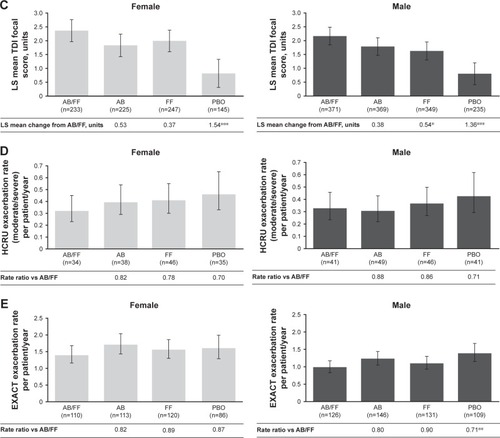
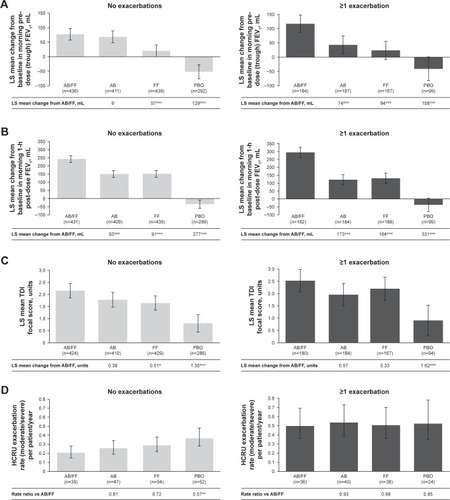
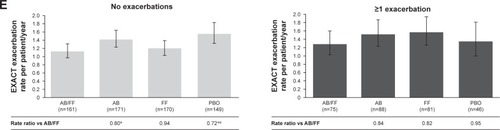
Figure 4 Efficacy endpoints at week 24 analyzed by prior exacerbation history (0 vs ≥1).
Abbreviations: AB, aclidinium bromide; EXACT, EXAcerbations of Chronic pulmonary disease Tool; FF, formoterol fumarate; HCRU, healthcare resource utilization; ITT, intent-to-treat; LS, least squares; PBO, placebo; TDI, Transition Dyspnea Index.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
