Figures & data
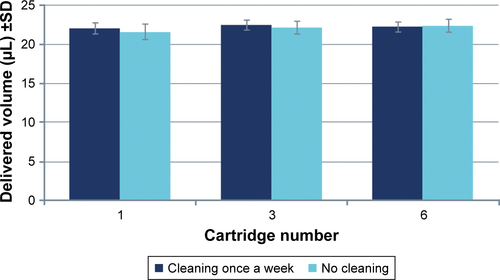
Table 1 Operating elements of Respimat and reusable Respimat
Figure 1 Respimat design features.
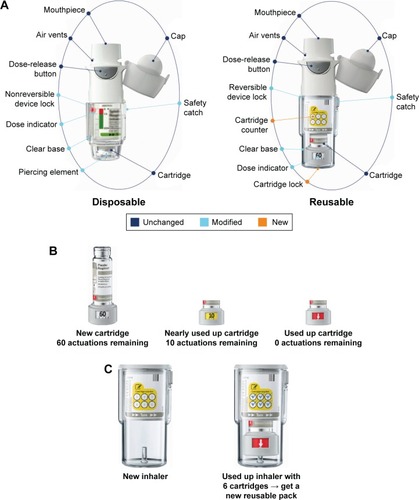
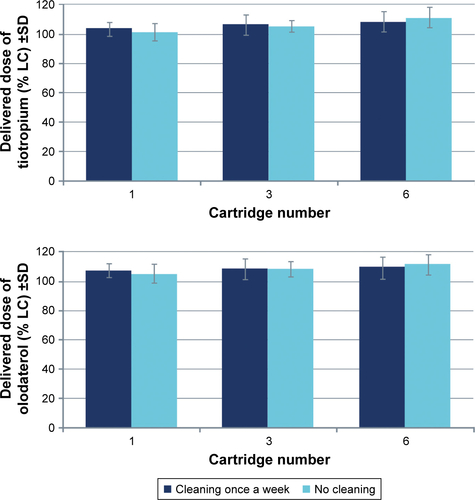
Figure 2 Delivered dose uniformity of (A) olodaterol and (B) tiotropium for nine cartridges in the reusable Respimat.
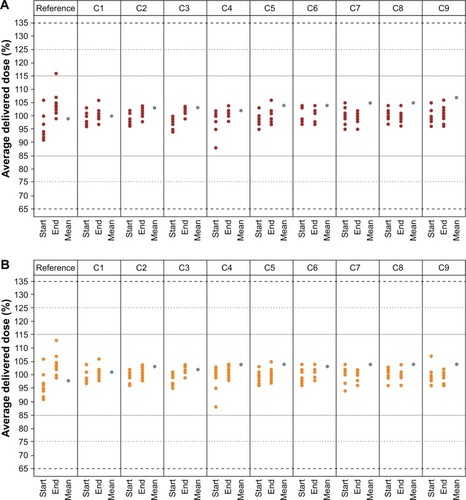
Figure 3 Fine-particle fraction for use of nine cartridges of tiotropium–olodaterol in the reusable Respimat inhaler determined by laser diffraction.
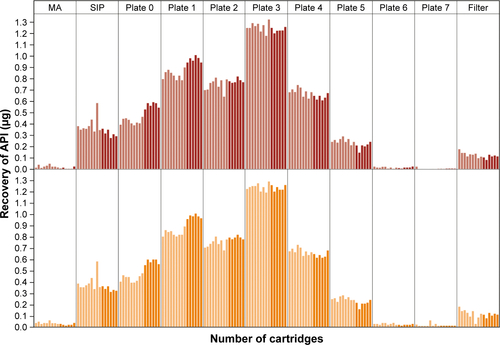
Notes: Gray bars represent a disposable inhaler batch as reference. Dark-blue bars represent the reusable inhaler used with nine cartridges. Mean values for individual inhalers (n=3) are shown. Correlating cutoff sizes of the Andersen cascade impactor were (µm): stage 0, >9.0; stage 1, 9.0–5.8; stage 2, 5.8–4.7; stage 3, 4.7–3.3; stage 4, 3.3–2.1; stage 5, 2.1–1.1; stage 6, 1.1–0.7; stage 7, 0.7–0.4; and filter, <0.4.
Abbreviation: SIP, sample induction port.
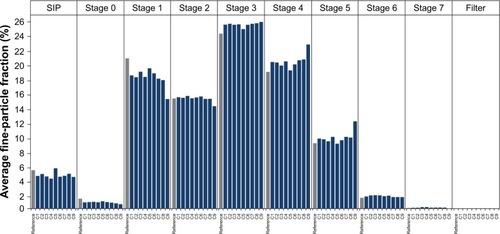
Figure 4 Aerodynamic particle-size distribution for use of nine cartridges of tiotropium–olodaterol in the reusable Respimat inhaler determined by the Andersen cascade impactor.
Abbreviations: LC, label claim; MA, mouthpiece adapter; SIP, sample induction port.
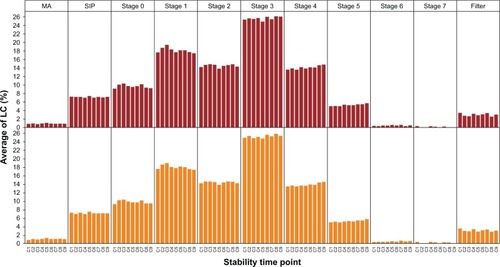
Figure 5 Overall perceived ease of performing cartridge insertion.
Note: Scoring from −7 (favors disposable Respimat) to +7 (favors reusable Respimat).
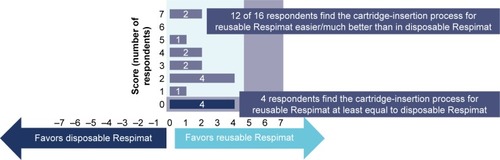
Figure 6 Rating of the clear base-detaching mechanism.
Note: Scoring from 1 (does not apply at all) to 7 (applies completely).
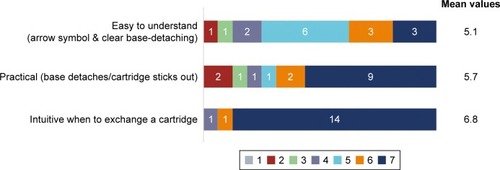
Figure 7 Rating of ease of exchanging a refill cartridge.
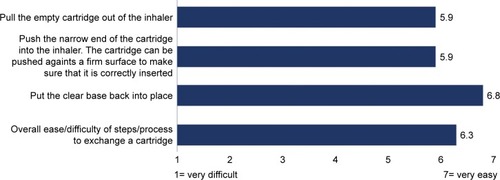
Figure S1 Particle-size distributions of tiotropium, olodaterol, and tiotropium–olodaterol by (A) laser diffraction and (B) Andersen cascade impactor.
Notes: Data are means of stability batches. For tiotropium–olodaterol Respimat, the particle-size distribution of tiotropium and olodaterol is shown. Correlating cutoff sizes of the Andersen cascade impactor were (µm): stage 0, >9.0; stage 1, 9.0–5.8; stage 2, 5.8–4.7; stage 3, 4.7–3.3; stage 4, 3.3–2.1; stage 5, 2.1–1.1; stage 6, 1.1–0.7; stage 7, 0.7–0.4; and filter, <0.4.
Abbreviations: Olo, olodaterol; SIP, sample induction port; T/O, tiotropium–olodaterol; Tio, tiotropium.
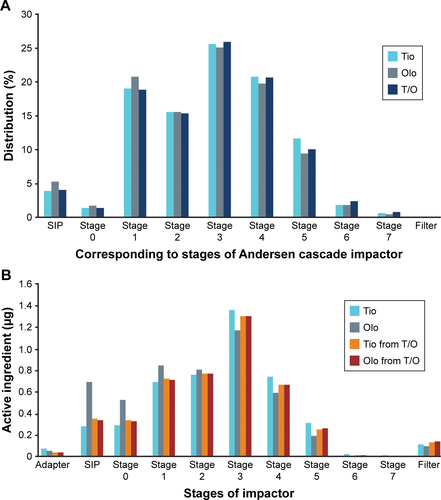
Figure S2 Delivered dose uniformity of (A) olodaterol and (B) tiotropium within reusable tiotropium–olodaterol Respimat for 15 cartridges.
Notes: Lighter columns, cartridges 1–9 (in-use testing); darker columns, cartridges 10–15 (sequential testing after 12 months’ nonuse). For each cartridge, the individual values at the start and the end of the testing schedule are given as a percentage referring to the average of all individual values, and the mean value is given as a percentage referring to the target dose.
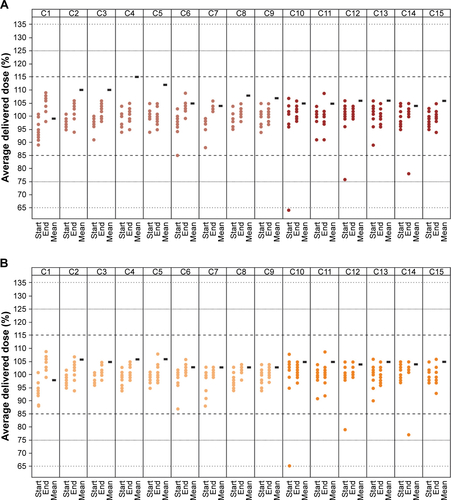
Figure S3 Particle-size distribution (Andersen cascade impactor) of tiotropium–olodaterol reusable Respimat for 15 cartridges.
Notes: Lighter columns, cartridges 1–9; darker columns, cartridges 10–15; upper panel, olodaterol; lower panel, tiotropium. Cutoff sizes of the Andersen cascade impactor were (µm): stage 0, >9.0; stage 1, 9.0–5.8; stage 2, 5.8–4.7; stage 3, 4.7–3.3; stage 4, 3.3–2.1; stage 5, 2.1–1.1; stage 6, 1.1–0.7; stage 7, 0.7–0.4; and filter, <0.4.
Abbreviations: API, active pharmaceutical ingredient; MA, mouthpiece adapter; SIP, sample induction port.