Figures & data
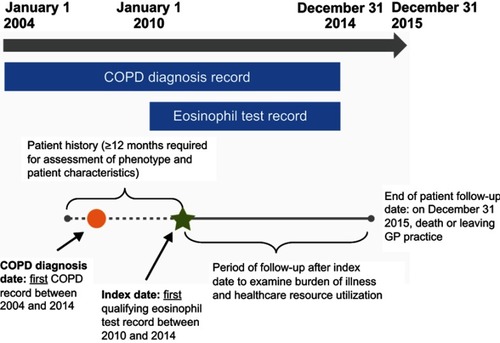
Figure 1 Proportion of patients meeting each defining criterion of the study phenotype, Numbers of patients meeting each criterion were as follows: EOS: n=19,303 (41.2%); MITT: n=2504 (5.3%); AECOPD: n=2094 (4.5%); MITT/EOS: n=4,845 (10.3%); AECOPD/EOS: n=3923 (8.4%); AECOPD/MITT: n=1408 (3.0%); Study phenotype: n=2512 (5.4%); no criteria: n=10,225 (21.8%). AECOPD, ≥2 moderate or ≥1 severe acute exacerbation of COPD in the 12 months prior to the index date; EOS, blood eosinophil count ≥150 cells/µL on the index date; MITT, multiple-inhaler triple therapy use on the index date. Study phenotype is defined as patients with ≥2 moderate or ≥1 severe acute exacerbation of COPD in the 12 months prior to the index date, who were receiving multiple-inhaler triple therapy at the index date, and who had a peripheral blood eosinophil count ≥150 cells/µL recorded on the index date.
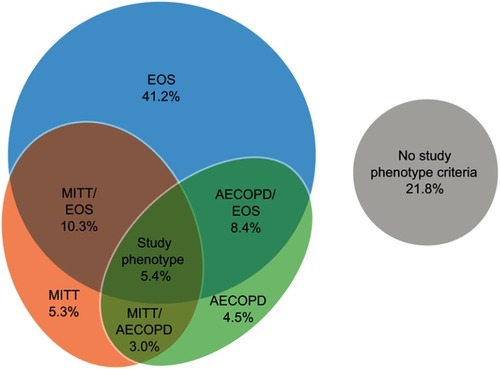
Figure 2 Unadjusted rates of clinical burden of illness outcomes during follow-up for patients with and without the study phenotype. Study phenotype is defined as patients with ≥2 moderate or ≥1 severe AECOPD in the 12 months prior to the index date, who were receiving multiple-inhaler triple therapy at the index date, and who had a peripheral blood eosinophil count ≥150 cells/µL recorded on the index date. Patients who did not meet all three of the criteria were classified as being in the non-study phenotype.
Abbreviation: AECOPD, acute exacerbation of COPD.
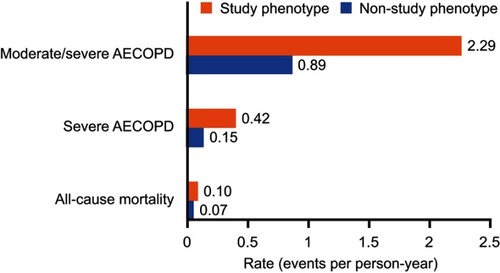
Table 1 Baseline demographic and clinical characteristics of patients with and without the study phenotype
Figure 3 Adjusted rate ratios of clinical burden of illness outcomes and hazard ratios for all-cause mortality for (A) patients with vs without the primary study phenotype, (B) patients with vs without study phenotypeS1, and (C) patients with vs without study phenotypeS2. Primary study phenotype is defined as patients with ≥2 moderate or ≥1 severe AECOPD in the 12 months prior to the index date, who were receiving multiple-inhaler triple therapy at the index date, and who had a peripheral blood eosinophil count ≥150 cells/µL recorded on the index date. For study phenotypeS1, the peripheral blood eosinophil count required on the index date was increased to ≥300 cells/µL. For study phenotypeS2, patients were required to have been receiving continuous multiple-inhaler triple therapy for ≥12 months prior to the index date. For each analysis, patients who did not meet all three of the criteria were classified as being in the non-study phenotype. Rate ratios adjusted for age, sex, geographical region in England, body mass index, smoking status, comorbidities, Index of Multiple Deprivation (twentiles, modeled as a continuous variable), primary care consultations in prior year, and season of index date. Rate ratios were calculated for all outcomes except for all-cause mortality where a hazard ratio was calculated.
Abbreviation: AECOPD, acute exacerbation of COPD.
Figure S2 Patient flow diagram. Study phenotype is defined as patients with ≥2 moderate or ≥1 severe AECOPD in the 12 months prior to the index date, who were receiving multiple inhaler triple therapy at the index date, and who had a peripheral blood eosinophil count ≥150 cells/µL recorded on the index date. Patients who did not meet all three of the criteria were classified as being in the non-study phenotype.
Abbreviations: AECOPD, acute exacerbation of COPD; CPRD, clinical practice research datalink; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HES, hospital episode statistics; IMD, Index of Multiple Deprivation.

Figure S3 Unadjusted rates of healthcare resource utilization outcomes during follow-up for patients with and without the study phenotype. Study phenotype is defined as patients with ≥2 moderate or ≥1 severe AECOPD in the 12 months prior to the index date, who were receiving multiple inhaler triple therapy at the index date, and who had a peripheral blood eosinophil count ≥150 cells/µL recorded on the index date. Patients who did not meet all three of the criteria were classified as being in the non-study phenotype.
Abbreviations: AECOPD, acute exacerbation of COPD; GP, general practitioner.
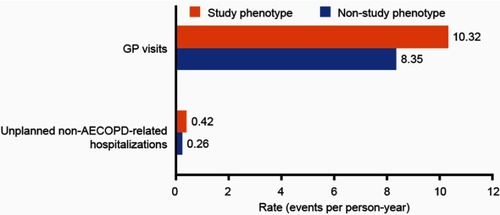
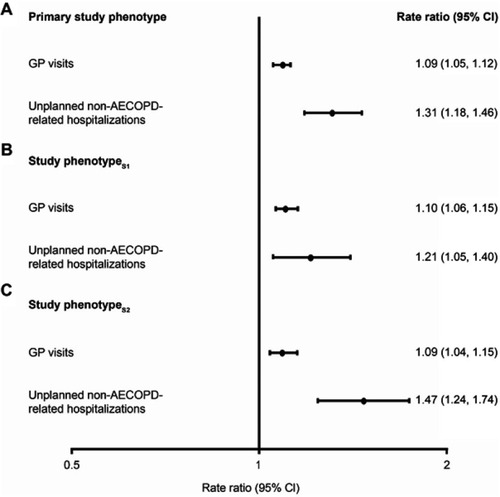
Figure S4 Adjusted rate ratios of healthcare resource utilization outcomes during follow-up for (A) patients with versus without the primary study phenotype, (B) patients with versus without study phenotypeS1, and (C) patients with versus without study phenotypeS2. Primary study phenotype is defined as patients with ≥2 moderate or ≥1 severe AECOPD in the 12 months prior to the index date, who were receiving multiple inhaler triple therapy at the index date, and who had a peripheral blood eosinophil count ≥150 cells/µL recorded on the index date. For study phenotypeS1, the peripheral blood eosinophil count required on the index date was increased to ≥300 cells/µL. For study phenotypeS2, patients were required to have been receiving continuous multiple inhaler triple therapy for ≥12 months prior to the index date. For each analysis, patients who did not meet all three of the criteria were classified as being in the non-study phenotype. Rate ratios adjusted for age, sex, geographical region in England, body mass index, smoking status, comorbidities, Index of Multiple Deprivation (twentiles modeled as a continuous variable), primary care consultations in prior year, and season of index date. Rate ratios were calculated for all outcomes.
Abbreviations: AECOPD, acute exacerbation of COPD; GP, general practitioner.
Data availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website. The data that support the findings of this study are available from UK CPRD and HES, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.