Figures & data
Table 1 Baseline characteristics (intention-to-treat population)
Table 2 Number (%) of patients with a CID overall and by individual component
Figure 1 TRILOGY: Time to (A) first CID and (B) sustained CID (without TDI).
Abbreviations: CID, clinically important deterioration; BDP, beclometasone dipropionate; FF, formoterol fumarate; G, glycopyrronium; TDI, Transition Dyspnea Index; SGRQ, St George’s Respiratory Questionnaire.
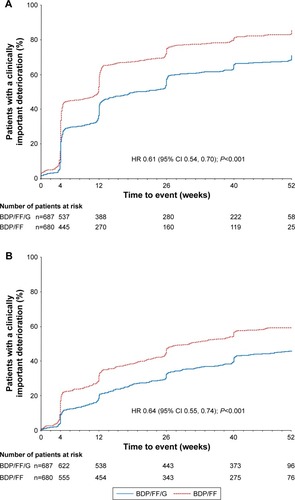
Figure 2 TRILOGY: Time to (A) first CID and (B) sustained CID (with TDI).
Abbreviations: CID, clinically important deterioration; BDP, beclometasone dipropionate; FF, formoterol fumarate; G, glycopyrronium; TDI, Transition Dyspnea Index; SGRQ, St George’s Respiratory Questionnaire.
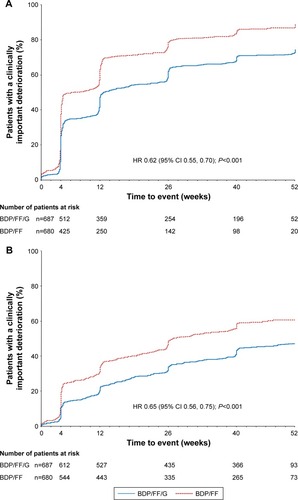
Figure 3 TRINITY: Time to (A) first CID and (B) sustained CID.
Abbreviations: CID, clinically important deterioration; BDP, beclometasone dipropionate; FF, formoterol fumarate; G, glycopyrronium; SGRQ, St George’s Respiratory Questionnaire.
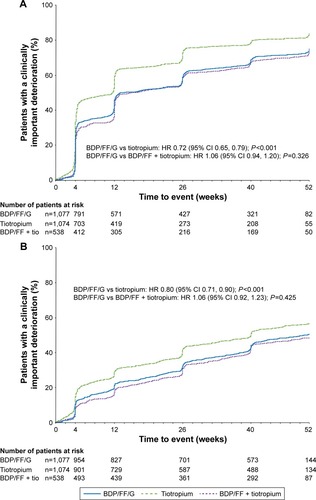
Figure 4 TRIBUTE: Time to (A) first CID and (B) sustained CID.
Abbreviations: CID, clinically important deterioration; BDP, beclometasone dipropionate; FF, formoterol fumarate; G, glycopyrronium; IND/GLY, indacaterol/glycopyrronium; SGRQ, St George’s Respiratory Questionnaire.
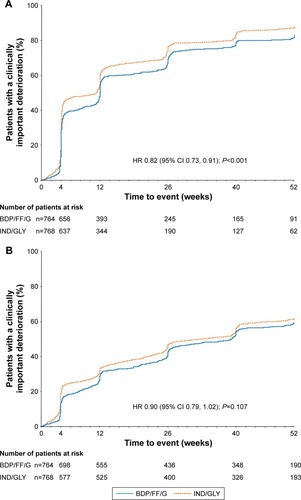
Figure S1 TRILOGY: Time to sustained CID (without TDI), alternative definition.
Notes: Time to sustained CID was defined as: a CID in FEV1 and/or SGRQ total score maintained at all subsequent visits; a moderate/severe exacerbation followed by a CID in FEV1 and/or SGRQ total score at all subsequent visits, or the exacerbation resulted in study discontinuation, or they had at least one further exacerbation; or death.
Abbreviations: CID, clinically important deterioration; BDP, beclometasone dipropionate; FF, formoterol fumarate; G, glycopyrronium; TDI, Transition Dyspnea Index; SGRQ, St George’s Respiratory Questionnaire.
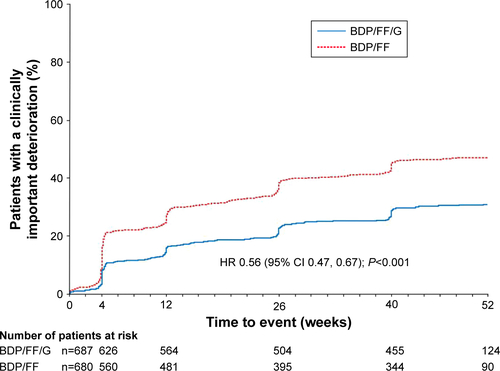
Figure S2 TRILOGY: Time to sustained CID (with TDI), alternative definition.
Notes: Time to sustained CID was defined as: a CID in FEV1 and/or SGRQ total score and/or TDI focal score maintained at all subsequent visits; a moderate/severe exacerbation followed by a CID in FEV1 and/or SGRQ total score and/or TDI focal score at all subsequent visits, or the exacerbation resulted in study discontinuation, or they had at least one further exacerbation; or death.
Abbreviations: CID, clinically important deterioration; BDP, beclometasone dipropionate; FF, formoterol fumarate; G, glycopyrronium; TDI, Transition Dyspnea Index; SGRQ, St George’s Respiratory Questionnaire.
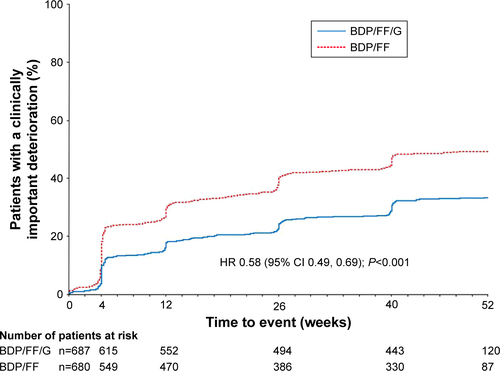
Figure S3 TRINITY: Time to sustained CID, alternative definition.
Notes: Time to sustained CID was defined as: a CID in FEV1 and/or SGRQ total score maintained at all subsequent visits; a moderate/severe exacerbation followed by a CID in FEV1 and/or SGRQ total score at all subsequent visits, or the exacerbation resulted in study discontinuation, or they had at least one further exacerbation; or death.
Abbreviations: CID, clinically important deterioration; BDP, beclometasone dipropionate; FF, formoterol fumarate; G, glycopyrronium; SGRQ, St George’s Respiratory Questionnaire.
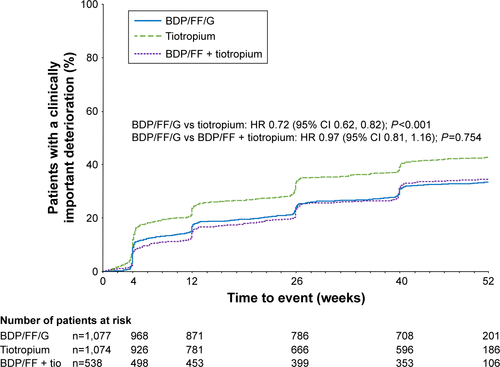
Figure S4 TRIBUTE: Time to sustained CID, alternative definition.
Notes: Time to sustained CID was defined as: a CID in FEV1 and/or SGRQ total score maintained at all subsequent visits; a moderate/severe exacerbation followed by a CID in FEV1 and/or SGRQ total score at all subsequent visits, or the exacerbation resulted in study discontinuation, or they had at least one further exacerbation; or death.
Abbreviations: CID, clinically important deterioration; BDP, beclometasone dipropionate; FF, formoterol fumarate; G, glycopyrronium; IND/GLY, indacaterol/glycopyrronium; SGRQ, St George’s Respiratory Questionnaire.
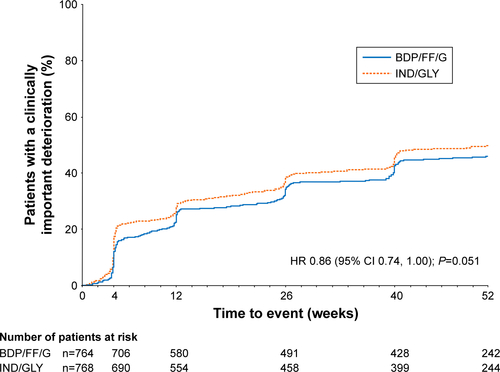
Table S1 Number (%) of patients with a sustained CID overall and by individual component
Data availability
Chiesi commits to sharing with qualified scientific and medical researchers, conducting legitimate research, the anonymized patient-level data, the study-level data, the clinical protocol and the full CSR of Chiesi Farmaceutici SpA-sponsored interventional clinical trials in patients for medicines and indications approved by the European Medicines Agency and/or the US Food and Drug Administration after 1 January 2015.
Chiesi commits to sharing the clinical data of TRILOGY (NCT1917331) and TRINITY (NCT1911364) studies starting from 1 January 2019, following the approval of any received research proposal and the signature of a Data Sharing Agreement. Chiesi provides access to clinical trial information consistently with the principle of safeguarding commercially confidential information and patient privacy. To date, TRIBUTE (NCT2579850) is out of scope of the Chiesi policy on clinical data sharing.
Other information on Chiesi’s data sharing commitment, access, and research request’s approval process will be available from 1 January 2019 in the Clinical Trial Transparency section of this webpage page: http://www.chiesi.com/en/research-and-development/.
