Figures & data
Figure 1 Flow chart depicting selection of sample and study cohorts. aFull coverage and COPD diagnosis <1 year prior to first nebulized LABA claim. Excluded deaths before first nebulized LABA claims.
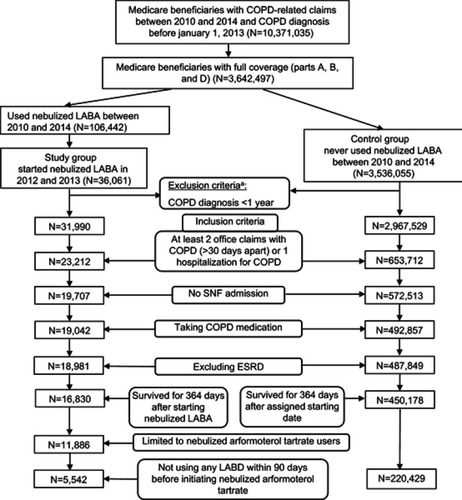
Table 1 Sociodemographic characteristics of study cohorts
Table 2 Comorbidities and types of COPD medications filled by Medicare beneficiaries 90 days before initiating nebulized arformoterol by (N=11,886)
Figure 2 Number of long-acting and short-acting bronchodilators filled by Medicare beneficiaries 90 days before initiating nebulized arformoterol (N=11,886). With the exception of beneficiaries taking 0 medication, no other categories are mutually exclusive (ie, categories refer to taking at least 1, 2, or 3 therapeutic agents).
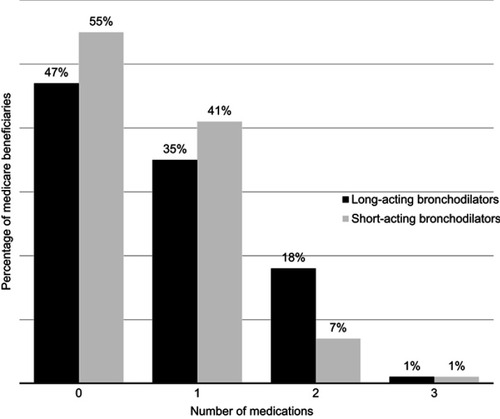
Figure 3 Medication use by Medicare beneficiaries 90 days before and after initiating nebulized arformoterol (N=11,886). Percentages are not mutually exclusive.
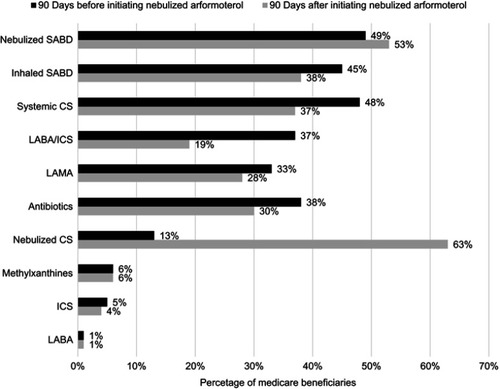
Figure 4 COPD medication patterns 90 days before initiating nebulized arformoterol among Medicare beneficiaries who received no long-acting bronchodilators (N=5,542). Percentages are not mutually exclusive.
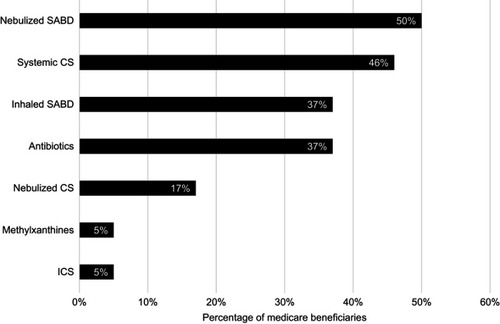
Table 3 Treatment characteristics of Medicare beneficiaries who received no long-acting bronchodilators 90 days before initiating nebulized arformoterol compared with controls
Table 4 Predictors associated with initiating nebulized arformoterol among Medicare beneficiaries who received no long-acting bronchodilators 90 days prior (N=5,542) compared with controls (N=220,429)
