Figures & data
Figure 1 Study design.
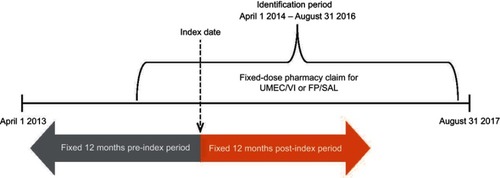
Table 1 Pre-index patient demographics and clinical characteristics before and after inverse probability of treatment weighting
Figure 2 Patient identification and attrition.
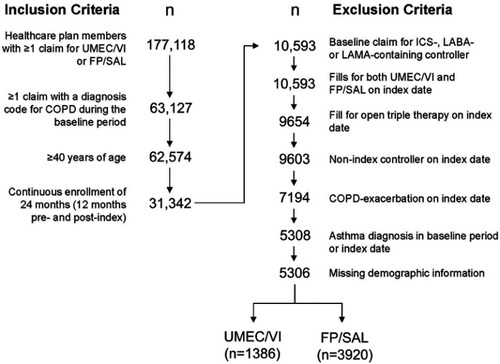
Figure 3 Medication adherence. (A) Mean PDC. (B) Percentage of patients achieving PDC≥80.
Abbreviations: FP/SAL, fluticasone propionate/salmeterol; PDC, proportion of days covered; UMEC/VI, umeclidinium/vilanterol.

Figure 4 Kaplan–Meier curves for incidence of moderate/severe exacerbations. (A) Intent-to-treat analysis. (B) On-treatment sensitivity analysis.
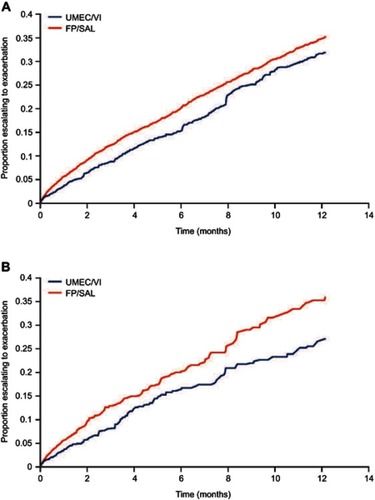
Figure 5 Kaplan–Meier curve for incidence of multiple-inhaler triple therapy.
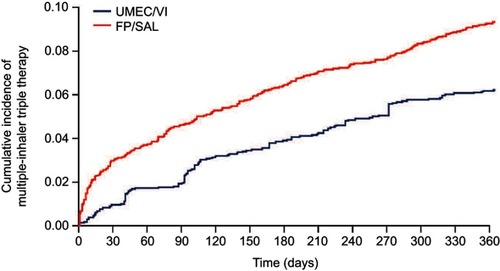
Table S1 Variables included in inverse probability of treatment weighting model
Table S2 Post-inverse probability of treatment weighting post-index time to first occurrence of multiple-inhaler triple therapy (intent-to-treat) – proportional hazard model, first 90 days and 91─365 days
Table S3 Secondary outcome: post-inverse probability of treatment weighting post-index time to first occurrence of multiple-inhaler triple therapy (intent-to-treat) – proportional hazard model, monthly time increments
