Figures & data
Figure 2 Prevalence of COPD according to different criteria (post-bronchodilator FEV1/FVC <0.70 and post-bronchodilator LLN for FEV1/FVC) using the Hospital Maciel, Montevideo, sample and the PLATINO study baseline population. Abbreviation: LLN, lower limit of normal.
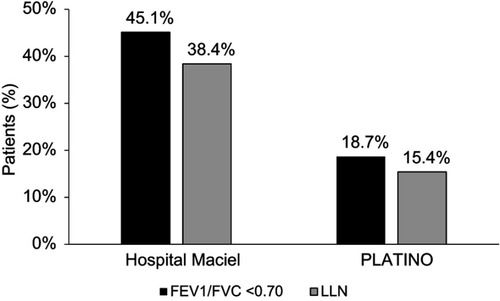
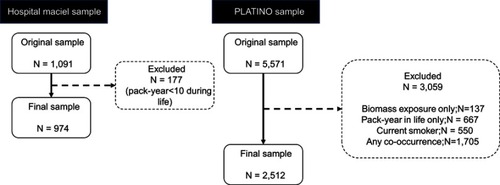
Table 1 Description of sample characteristics for the Hospital Maciel, Montevideo, and the PLATINO study samples
Table 2 Description of sample characteristics for the Hospital Maciel, Montevideo, and the PLATINO study samples in individuals with COPD defined using the post-bronchodilator FEV1/FVC <0.70 definition
Table 3 Description of sample characteristics for the Hospital Maciel, Montevideo, and the PLATINO study samples in individuals with COPD defined using the post-bronchodilator LLN FEV1/FVC definition
Table 4 Sensitivity, specificity, PPV, PNV for each cut-point of proposed score (1 point for each category variable) using Hospital Maciel, Montevideo, and PLATINO study samples
Figure 3 Area under the ROC for score (1 point for each category variable) with COPD as outcome using: (A) post-bronchodilator FEV1/FVC <0.70 and (B) post-bronchodilator LLN for FEV1/FVC as definitions for the Hospital Maciel, Montevideo, the PLATINO study, and the original PUMA study baseline populations as samples.

Table S1 Points applied for each variable in the PUMA questionnaire